IRINOTECAN Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
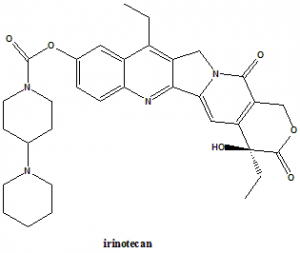
Irinotecan
IUPAC nomenclature
(S)-4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxo1H-pyrano[3’,4’:6,7]-indolizino[1,2-b]quinolin-9-yl-[1,4’bipiperidine]-1’-carboxylate.
Classification
Irinotecan falls under the category of camptothecin cytotoxic drug. [1]
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 586.7g/mol |
| 2 | Appearance | Pale yellow powder |
| 3 | Melting point | Range between 222-223°C |
| 4 | Solubility | Soluble in water |
| 5 | Octanol water partition coefficient | 3.2 |
| 6 | Presence of ring | Ring structures like pyridine and piperidine are present. |
Mechanism of Action
i. During the S phase of the DNA synthesis, Irinotecan binds with the topoisomerase I-DNA complex. This prevents the relegation of the DNA strands. .
ii. The ternary complex so formed interferes with the moving replication fork.
iii. The lethal double-stranded breaks in the mammalian cells cannot be repaired by the cells and thus apoptosis takes place. [2]
Structural Activity Relationship
- The E ring in a lactone form is much more potent than the E-ring in the caboxylate form.
- For the activity of the drug, chiral center at position 20 is necessary.
- R-configuration is inactive form.
- Drug without the A and B rings shows less potency for the DNA synthesis inhibition.
- A and B rings are important for the activity of the drug. [3]
Method of Synthesis
i. Synthesis of preparing 10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptotecin from the campthothecin.
ii. Selectively ethylating the compound at the 7- position.
iii. 10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptothecin (i.e. 7-des-ethyl-irinotecan) is so formed as an intermediate is then converted to irinotecan.
Therapeutic Uses
Irinotecan is used for the treatment of metastatic colon and rectal cancer.
Side Effects
- Common side effects includes diarrhea, nausea, vomiting, weakness, low blood count, hair loss, poor appetite, fever and weight loss.
- Some people may suffer from side effects like constipation, shortness of breath, insomnia, cough, headache, dehydration, chills, skin rashes, flatulence, mouth sores, swelling of feet and ankles and heart burns.
MCQs
Q.1 The term Camptosar is associated with which drug?
a) Methotrexate
b) Doxorubcin
c) Irinotecan
d) Topotecan
Q.2 The correct statement related with the SAR of drug Irinotecan is-
a) The E ring in a carboxylate form is much more potent than the E-ring in the lactone form.
b) For the activity of the drug, chiral center at position 20 is not necessary
c) A and B rings are not important for the activity of the drug.
d) The E ring in a lactone form is much more potent than the E-ring in the caboxylate form.
Q.3 Which amongst the following are the correct side effects of the drug Irinotecan?
I. Weight gain
II. Increase in the blood count
III. Hair loss
IV. Shortness of breath
a) I, III & IV
b) Only III & IV
c) I & III only
d) All
Q.4 The starting chemicals required for the synthesis of drug Irinotecan is?
a) Morphine
b) Epipodophyllotoxin
c) Camptothecin
d) Streptomycin
Q.5 Which pairs of drug and its classification are true
| I. | Topotecan | Nitrosoureas |
| II. | Irinotecan | Camptothecin |
| III. | Etoposide | Epipodophyllytoxin |
| IV. | Dacarbazine | Triazine |
a) II, III & IV
b) I, III & IV
c) I & II
d) III & IV
Q.6 Correct physical form in which the drug Irinotecan is found?
a) Pale yellow powder
b) Transparent crystalline form
c) White Crystalline form
d) Yellow oily liquid
Q.7 Match the following with respect to ring systems of the drug
| i. Irinotecan | A. Pyrimidine ring system |
| ii. 6-MP | B. Piperidine ring structure |
| iii. 5-FU | C. Purine ring system |
| iv. Methotrexate | D. Pteridine ring system |
a) i-C, ii-D, iii-B, iv-A
b) i-B, ii-C, iii-A, iv-D
c) i-A, ii-B, iii-D, iv-C
d) i-C, ii-B, iii-A, iv-D
ANSWERS
1-c
2-d
3-b
4-c
5-a
6-a
7-b