MASS SPECTROSCOPY: Principle, Theory, Instrumentation and MCQ for GPAT, GATE
MASS SPECTROSCOPY is an analytical technique that measures the mass-to-charge ratio of charged particles. It is used for determine masses of particles, for determining the elements composition of a sample or molecules and for elucidating the chemical structures of molecules.
PRINCIPLE :-
Mass spectra is also called as positive ion spectra or line spectra. we donot use any Electro magnetic radiation for excitation. obtaining mass spectra consists of 5 steps:-
- molecule are bombared with electron
- Converted into highly positive charged ions
- further break up into smaller ions
- The formed ions are separated by deflection in magnetic field
- According to their mass &charge and get mass spectrcm
THEORY :-
The relative abundance of positively charged fragments of various mass to charge ratio is the characteristics feature of the molecules that serve to identify the substance. It is determine by
½ MV2 = ev {Kinetic energy = 1/2MV2}
Voltage
r=(2V\H2 m/e) 1/2
INSTRUMENTATION:-
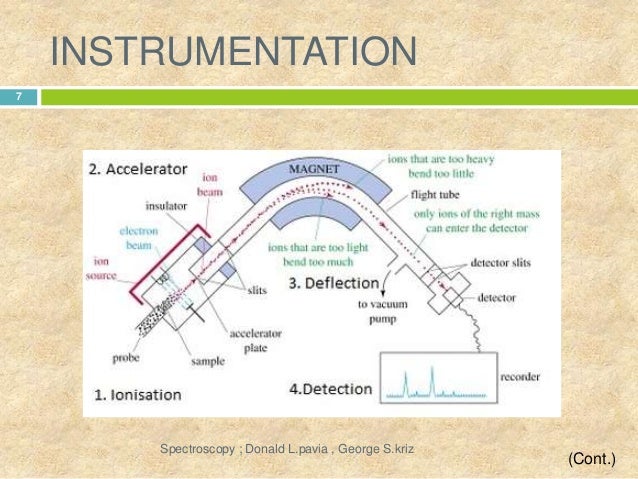
- SAMPLE INLET:- Sample stored in large reservoir from which molecules reaches ionization chamber at low pressure in steady stream by a pinhole called “molecular leak”.
- IONIZATION :- Atoms ionized by knocking one or more electrons off to give positive ions by bombaredment with a stream of electron. most of the positive ions formed will carry charge of +1 or +2.
- ACCELERATION :- Ions accelerated so that they all have same kinetic energy. +ve ions pass through 3 slits with voltage in decreasing order. middle slit carries intermediate and finals at zero volts. All ions are accelerated into finaly forward beam.
- DEFLECTION :- Ions are deflected by a magnetic field due to difference in their masses. the lighter mass more they are deflected. It also depends upon the no. of +ve charge an ion is carrying the more +ve charge more it will be deflected.
- DETECTION:- The beam of ions passing through the mass analyzer are detector on the basis of m/z ratio.
IN A TYPICAL MS PROCEDURE :-
- A sample is loaded into the MS instrument and undergoes vaporization
- The components of the sample are ionized by one of a variety of methods (e.g. by impacting them with an electron beam) which result in the formation of charged particles.
- The ions are separated according to their mass to charge ratio in an analyzer by electromagnetic fields.
- The ions are detected usually by a quantitative method.
- The ion signal is processed into mass spectra.
MCQ
1.what is use of mass spectroscopy ?
A.Determination of molecule weight
B.Elucidating the chemical structures of molecules
C. A and B
D.None of the above
2. Mass spectra is also called
A. Positive ion spectra
B.Line spectra
C. A and B
D.None of the above
3. Highest m/z peak in mass spectrum is called as
A. Base peak
B.Fragment peak
C.Isotopic peak
D.Parent peak
4.Mass spectrometer requires
A.High temprature
B.High cooling
C. High vaccume
D.High pressure
5. Which ratio is measured by mass detector ?
A.z/m
B.e/m
C.m/e
D. m/z
6. Chosse the correct sequence of ms procedure ?
p.The ion signal is processed into mass spectra.
q.The ions are detected usually by a quantitative method.
r.The ions are separated according to their mass to charge ratio in an analyzer by electromagnetic fields.
s.The components of the sample are ionized by one of a variety of methods (e.g. by impacting them with an electron beam) which result in the formation of charged particles.
w.A sample is loaded into the MS instrument and undergoes vaporization
A.p→q→r→s→w
B.w→s→r→q→p
C.p→w→r→s→q
D.q→w→r→s→p
ANSWER KEY:-
1.C
2.C
3.A
4.C
5.D
6.B
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
REFERENCE :-
TEXT BOOK OF PHARMACEUTICAL ANALYSIS THIRD EDITION OF Dr.s.ravi sankar (pg.no.8.1 to 8.5)
TEXT BOOK OF PHARMACEUTICAL ANALYSIS FOURTH EDITION DAVID G WASTON (PG.NO. 200 TO 244)