Matter, properties of matter: Latent heat and vapour pressure, sublimation – critical point, eutectic mixtures and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam
Latent heat The quantity of heat absorbed or released by a substance undergoing a change of state. Also called heat of transformation. In the case of water, 1 gram of ice at 0⁰C requires 336 joules of energy to convert it to 1g of water at 0⁰C (latent heat of fusion). As matter change from higher kinetic energy to lower one (e.g. stream to water) this latent energy is released. The concept of latent energy has practical applications: Ice milting on the skin takes considerable energy(heat) from the skin, thus cooling it , whereas paraffin wax solidifying on the skin gives out considerable heat to the skin, thus warming it.
Vapour pressure is defined as the pressure exerted by a vapour in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The equilibrium vapour pressure is an indication of a liquid’s evaporation rate. It relates to the tendency of particles to escape from the liquid (or a solid). A substance with a high vapour pressure at normal temperatures is often referred to as volatile. The pressure exhibited by vapour present above a liquid surface is known as vapour pressure.
In case of liquids, kinetic energy is not distributed evenly among molecules and some of the molecules acquire more energy and hence higher velocities than others. The molecules that have sufficient energy to overcome intermolecular attractions are able to escape from the surface into the vapour phase (gas phase). The process is known as evaporation.
The average kinetic energy of molecules in vapour state is more compared to molecules in liquid state and therefore the temperature of the liquid falls on evaporation. The rate of evaporation of a liquid depends upon the temperature of the liquid, surface area, pressure above the liquid and attractive forces in the liquid.
In an another process known as condensation, the molecules of liquid in the vapour phase undergo collisions among each other and with the sides of the evaporating still, transfer their energy to other molecules and come back to the liquid phase. The rate of condensation of molecules in the vapour phase is proportional to the concentration of molecules in the vapour phase. At dynamic equilibrium conditions, the rate of evaporation becomes equal to the rate of condensation.
The relationship between the vapour pressure and the absolute temperature of the liquid is expressed by the
Clausius–Clapeyron equation:
log p2/p1 = ‘H (T2 – T1)/ 2.303 RT1T2
where, ‘H is the molar heat of vaporization, p1 and p2 are the vapour pressure at absolute temperatures T1 and T2.
As the temperature of a liquid increases, the kinetic energy of its molecules also increases. As the kinetic energy of the molecules increases, the number of molecules transitioning into a vapour also increases, thereby increasing the vapour pressure. As the temperature is raised further, the density of the vapour increases while that of liquid decreases. Eventually, the densities of both the phases become equal and the two phases cannot be distinguished. The temperature at which this happens is known as the critical temperature and above this the temperature; there is no liquid-phase.
Boiling point – Boiling point is the temperature of the liquid at which, the vapour pressure becomes equal to the atmospheric pressure. At this temperature, a liquid changes its state from liquid to vapour. Since at a given pressure different liquids boil at different temperatures, the normal boiling point (also known as the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is considered to be taken at an atmospheric pressure at sea level (i.e. 1 atm or 760 mmHg). At the boiling point, all the heat absorbed is used to convert the liquid to the vapour state and there is no increase in the temperature of the liquid until it is completely vapourized. This heat is known as latent heat of vapourization.
Melting point and freezing point – The melting point of a substance is the temperature at which it changes its state from solid to liquid. During the melting process, all the energy provided to a substance is consumed as latent heat of fusion, and the temperature remains constant. At the melting point, the solid and liquid phases coexist in equilibrium. When considered as the temperature of the reverse change from liquid to solid state, it is referred to as the freezing point or crystallization point.
Vapour pressure of a mixture of liquids –
In the case of miscible liquids (solution of a liquid in liquid), the partial vapour pressure exerted by each components is proportional to its molar concentration in the mixture solution. The total vapour pressure P is given by :
P=PA+PB=PoAXA+PoBXB
When XA and XB are mole fraction of components A and B respectively
PoA & PoB are the vapour pressure exerted by the pure components A and B respectively.
PA and PB are the partial vapour pressure exerted by A and B respectively in the liquid mixture in the case of a mixture containing two miscible liquids each liquid exerts it own vapour pressure independently of the others. The total vapour pressure P is then given by:
P=PoA +PoB
Sublimation-critical point: Sublimation is the process where a solid changes from a solid to a vapor without passing through the liquid state. Sublimation is an endothermic phase transition that occurs at temperatures and pressures below a substance’s triple point in its phase diagram. In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) coexist in thermodynamic equilibrium. A phase transition in which a solid is converted to a gas, without passing though an intermediate liquid phase. Dry ice” is actually solid, frozen carbon dioxide, which happens to sublimate, or turn to gas, at a chilly -78.5 °C (-109.3°F). The fog you see is actually a mixture of cold carbon dioxide gas and cold, humid air, created as the dry ice “melts” it mean sublimates.
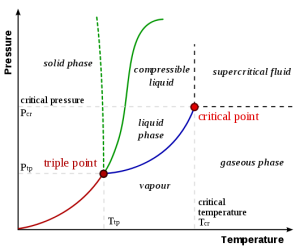
Fig. 1: Phase diagram illustrating the principle of sublimation (taken from Phase Diagrams – Chemistry Libre Texts)
Advantages of sublimation
1- The main advantage of sublimation is for purification process.
2- The minimum amount of product is loss.
3- Solvents are not used.
4- Most traces of any solvent in compound are effectively eliminated.
5- when the substance weighs less than 100mg the best method for purification is sublimation.
Sublimation is caused by the absorption of heat which provides enough energy for some molecules to overcome the attractive forces of their neighbors and escape into the vapor phase. Since the process requires additional energy, it is an endothermic change. The enthalpy of sublimation (also called heat of sublimation) can be calculated by adding the enthalpy of fusion and the enthalpy of vaporization.
Desublimation refers to the process in which a gas changes directly to a solid without going through the liquid state. Deposition as a change of state often occurs in nature. For example, when warm moist air comes into contact with very cold surfaces—such as the ground or objects on the ground—ice crystals are deposited on them. Deposition is used widely to create materials in industry, especially to apply a thin coating to materials used for cutting or shaping. Much research is ongoing in the field of chemical vapor deposition, especially in the area of materials used to cover polymers, and finding materials that are less harmful to the environment.
Eutectic mixtures: A eutectic mixture is defined as a mixture of two or more components which usually do not interact to form a new chemical compound but, which at certain ratios, inhibit the crystallization process of one another resulting in a system having a lower melting point than either of the components. Eutectic mixture formation is usually, governed by following factors:
(a)components must be miscible in liquid state and mostly immiscible in solid state
(b)Intimate contact between eutectic forming materials is necessary for contact induced melting point depression
(c)the components should have chemical groups that can interact to form physical bonds such has intermolecular hydrogen bonding etc.
(d)the molecules which are in accordance to modified VantHoff’s equation can form eutectic mixtures. Eutectic mixtures, can be formed between Active Pharmaceutical Ingredients (APIs), between APIs and excipients or between excipients; thereby providing a vast scope for its applications in pharmaceutical industry. During pre-formulation studies- Testing for eutectic mixture formation can help in anticipation of probable physical incompatibility between drug and excipient molecules. Eutectic mixtures are commonly used in drug designing and delivery processes for various routes of administration.
The coordinates defining a eutectic point on a phase diagram are the eutectic percentage ratio (on the atomic/molecular ratio axis of the diagram) and the eutectic temperature (on the temperature axis of the diagram).
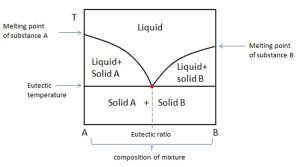
Fig 2: Phase diagram showing a eutectic system (taken from thermal energy storage EME 812: utility solar power and concentration)
In pharmaceutical practice, eutectic mixtures are difficult to dispense in the form of a powder. In order to incorporate such materials in a powder, it is essential to first mix each ingredient separately with an inert diluent such as light magnesium oxide, magnesium carbonate, starch, kaolin etc. followed by gentle blending of the different positions. Alternative the eutectic materials can first be triturated together in order to force them to liquify. The liquid can then be adsorbed on an inert diluents.
The phenomenon of eutectic formation has also been used in pharmaceutical practice to improve the dissolution behaviour of certain drugs. For example eutectic mixture of Aspirin – acetaminophen (37% and 63% respectively), urea – acetaminophen (46% and 54% respectively) and griseofulvin-succinic acid (55% and 45% respectively) dissolve rapidly than the drugs alone or their simple mixtures.
Multiple choice questions (MCQs)
1.The boiling point of a liquid is the temperature at which ______________ occurs throughout the liquid.
a)Freezing
b)Solidification
c)Sublimation
d)Evaporation
2.Dry ice is used in fire extinguishers. The dry ice is stored in the cylinder in a solid form. When sprayed on a fire it quickly changes into the gas known as carbon dioxide(CO2).
What is this change of state called?
a)Sublimation
b)Condensation
c)Distillation
d)Evaporation
3.What is vaporization?
a)A gas becoming a liquid
b)A liquid becoming a solid
c)A gas becoming a solid
d)A liquid becoming a gas
4.During the process of sublimation,
a)a solid turns directly into gas
b)a solid turns into a liquid
c)a gas turns directly into a solid
d)a liquid turns into a gas
5.An uncovered pot of soup is simmering on a stove, and there are water droplets on the wall above the back of the stove. What sequence can you infer has occurred?
a)Melting, then boiling
b)Freezing, then thawing
c)Vaporization, then condensation
d)Condensation, then vaporization
6.When a person wearing glasses enters a warm house after being outside on a cold day, their glasses fog up because of
a)Vaporization
b)Condensation
c)Sublimation
d)Melting
7.The opposite of vaporization is called
a)Condensation
b)Sublimation
c)Evaporation
d)Freezing
8.The process in which heat is required to convert a liquid into the vapour state is known as:
a)Evaporation
b)Latent heat of vaporization
c)Melting
d)Condensation
9.A substance with a high vapour pressure at normal temperatures is often referred to as
a)volatile
b)non volatile
c)ideal solution
d)non ideal solution
10.Name the liquid with higher vapour pressure in the following pairs:
a)Alcohol, petrol. water
b)Petrol, kerosene
c)mercury, water
d)None of these
11.The temperature at which the densities of both the phases become equal and the two phases cannot be distinguished is called
a)critical temperature
b)eutectic point
c)sublimation
d)triple point
12.If the external pressure above the surface of a liquid is decrease or increase the boiling point of the liquid is also decreased or increased.
a)True
b)False
13.In the case of miscible liquids (solution of a liquid in liquid), the partial vapour pressure exerted by each components is proportional to its
a)molal concentration
b)molar concentration
c)normal concentration
d)all of the above
14.Sublimation is a/an
a)exothermic process
b)endothermic process
c)both of these
d)none of these
15.A homogeneous mixture of substances that melts or solidifies at a single temperature that is lower than melting point of the constituents is called
a)eutectic system
b)isolated system
c)closed system
d)open system
Solutions:
- d) evaporation
- a) sublimation
- d) a liquid becoming a gas
- a) a solid turns directly into gas
- c) vaporization, then condensation
- b) condensation
- a) condensation
- a) evaporation
- a)volatile
- c) mercury, water
- a)critical temperature
- a)True
- b)molar concentration
- b)endothermic process
- a)eutectic system
References:
1. GAURAV KUMAR JAIN – THEORY & PRACTICE OF PHYSICAL PHARMACY, 1st edition 2012 Elsevier, page no. 2-7.
2. Martins Physical Pharmacy, 6th edition 2011, page no. 57-61.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test