Matter: Relative humidity, liquid complexes, liquid crystals, glasses state and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam
Relative humidity: It is a ratio, expressed in percent, the amount of atmospheric moisture present relative to the amount that would be present if the air is saturated. Since the later amount is dependent on temperature. Relative humidity is a function of both moisture content and temperature. Relative humidity is derived from the associated temperature and dew point for the indicator hour.
Relative humidity is normally expressed as a percentage; a higher percentage means that the air–water mixture is more humid. At 100% relative humidity, the air is saturated and is at its dew point.
A hygrometer is a device used for measuring the humidity of air.
Liquid complexes: Complex fluids are binary mixtures that have coexistence between two phases: solid–liquid (suspensions or solutions of macromolecules such as polymers), solid–gas (granular), liquid–gas (foams) or liquid–liquid (emulsions). They exhibit unusual mechanical responses to applied stress or strain due to the geometrical constraints that the phase coexistence imposes. The mechanical response includes transitions between solid-like and fluid-like behavior as well as fluctuations. Their mechanical properties can be attributed to characteristics such as high disorder, caging, and clustering on multiple length scales. Shaving cream is an example of a complex fluid.
Their mechanical properties can be attributed to characteristics such as high disorder, caging, and clustering on multiple length scales.
Liquid crystals: Three states of matter, gas, liquid and solid, have been discussed thus far. A fourth state of matter is the liquid crystal state or mesophase. The liquid crystal state is a distinct state of matter observed between the crystalline solid and liquid states. In the crystalline solid state, the arrangement of molecules is regular. The molecules are held in fixed positions by intermolecular forces. As the temperature of a substance increases, its molecules vibrate and eventually these vibrations overcome the forces that hold the molecules in place and the molecules start to move. In the liquid state, this motion overcomes the intermolecular forces and the molecules move into random positions. n the liquid crystal state, the increased molecular motion overcomes the weaker forces, but molecules remain bound by the stronger forces. This produces a molecular arrangement where molecules are in layers, but within each layer, molecules are arranged in random positions, more or less parallel to each other. The molecules can slide around each other and the layers can slide over one another. This molecular mobility produces the fluidity in liquid crystal state.
Characteristics of Liquid Crystal State:
1. The molecules of liquid crystal point along a common axis. This is in contrast to liquid phase molecules, which have no intrinsic order, whereas solid state molecules are highly ordered.
2. Most liquid crystal compounds exhibit polymorphism.
3. They are anisotropic in nature and their properties depend upon the direction along which they are measured.
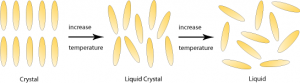
Fig 1 – Liquid crystals (taken from DoITPoMS – TLP library liquid crystals)
Types of Liquid crystals: Liquid crystals are
- Thermotropic Liquid Crystals – Liquid crystals are said to be thermotropic if liquid crystalline properties depend on the temperature.
- Nematic Liquid Crystals: Here the molecules (mesogens) have no positional order, but they have long-range orientational order
- Smectic Liquid Crystals: In this, the mesogens have both positional order and orientational order.
- Cholestric liquid Crystals: The cholestric phase can be defined as a special type of nematic liquid crystals in which thin layers of the parallel mesogens have their longitudinal axes rotated in adjacent layers at certain angle.
- Lyotropic Liquid Crystals: Liquid crystals which are prepared by mixing two or more substances, of which one is a polar molecule, are known as lyotropic liquid crystals.
Glasses State: The glassy state of materials refers to non equilibrium, solid state, such as is typical of inorganic glasses, synthetic noncrystalline polymers and food components. Characteristics of the glassy state include transparency, solid appearance and brittleness. In such systems, molecules have no ordered structure and the volume of the system is larger than that of crystalline systems with the same composition. These systems are often referred to as amorphous (i.e., disordered) solids (e.g., glass) or super cooled liquids (e.g., rubber, leather, syrup).
All the glass is considered to be a non-conducting transparent solid, it is actually a type of solid matter. It can neither be considered as a typical solid nor a typical liquid. The atoms and molecules in most solids are arranged in an orderly manner whereas in Glassy materials these are highly disorder. Glassy materials however, have some short range order as in the case of polymers. Glassy materials also do not have a specific melting point but these slowly and gradually liquefy on heating. Structurally Glassy materials can be considered to be made up of a random selection of polyhedral molecules linked together at their corners. Certain materials can easily be converted to a Glassy state while other pose great difficulty and certain materials cannot be converted at all. Although the theory behind this behaviour is not very clear, it has been shown that material which can be converted to glassy state have a very high viscosity at their melting point which inhibits the formation of an ordered structure Although the most common materials which can be converted to Glassy state are the metal oxides ,even materials such as Steel can be converted to the Glassy state if it is cooled very quickly. This technique produces glasses since the material solidifies even before it gets chances to develop a crystalline structure.
Glasses are generally formed by melting crystalline materials at very high temperatures. When the melt cools, the atoms are enclosed in a random (disordered) state before they can form in a perfect crystalline arrangement.
Types: There are three types of glassy states.
The first type: It is characterized by the cessation of the vibratory movement of rotation of the molecules in a defined (critical) temperature region. This results in stabilization of the chain structures of rigidly associated polar molecules (by means of dipoles).
The second type: It is consists of organic glassy polymerization products. These glass in the stabilized state have fibrous structure of rigid valence bonded carbon atoms with small lateral branches in the form of hydrogen atoms or more complex radicals.
The Third type: The third most extensive type of glassy state consists of refractory inorganic compounds of multivalent elements. These glasses in the stabilized state have the most thermostable chemical structure in the form of a three dimensional rigid atomic valency-bonded spatial network.
Multiple choice questions (MCQs)
1.Relative humidity is a ratio, expressed in
a)kg
b)millimole
c)ppm
d)percentage
2.Relative Humidity is derived from
a)Temperature
b)Dew Point
c)Both of these
d)None of these
3.At 100% relative humidity, the air is saturated and is at its dew point.
a)True
b)False
4.Which device is used for measuring the humidity of air?
a)Hydrometer
b)Hygrometer
c)Pycnometer
d)Viscometer
5.Which of the following is solid–liquid complex?
a)suspensions or solutions of macromolecules such as polymers
b)granular
c)foams
d)emulsions
6.Which of the following is solid–gas complex?
a)suspensions or solutions of macromolecules such as polymers
b)granular
c)foams
d)emulsions
7.Which of the following is liquid–gas complex?
a)suspensions or solutions of macromolecules such as polymers
b)granular
c)foams
d)emulsions
8.Which of the following is liquid–liquid complex?
a)suspensions or solutions of macromolecules such as polymers
b)granular
c)foams
d)emulsions
9.Which of the following are types of Liquid crystals?
a)Thermotropic Liquid Crystals
b)Lyotropic Liquid Crystals
c)Both of these
d)None of these
10.Liquid crystals are said to be thermotropic if liquid crystalline properties depend on
a)temperature
b)pressure
c)particle size
d)nature of crystal
11.Here the molecules (mesogens) have no positional order, but they have long-range orientational order
a)Nematic Liquid Crystals
b)Smectic Liquid Crystals
c)Cholestric liquid Crystals
d)All of these
12.In this, the mesogens have both positional order and orientational order.
a)Nematic Liquid Crystals
b)Smectic Liquid Crystals
c)Cholestric liquid Crystals
d)All of these
13.A special type of nematic liquid crystals in which thin layers of the parallel mesogens have their longitudinal axes rotated in adjacent layers at certain angle is known as
a)Nematic Liquid Crystals
b)Smectic Liquid Crystals
c)Cholestric liquid Crystals
d)All of these
14.Glasses are generally formed by melting crystalline materials at
a)High temperature
b)High pressure
c)Low temperature
d)Low pressure
15.Liquid crystals which are prepared by mixing two or more substances, of which one is a polar molecule, are known as
a)Nematic Liquid Crystals
b)Smectic Liquid Crystals
c)Cholestric liquid Crystals
d)Lyotropic Liquid Crystals
Solutions:
- d)percentage
- c)Both of these
- a)True
- b)Hygrometer
- a)suspensions or solutions of macromolecules such as polymers
- b)granular
- c)foams
- d)emulsions
- c)Both of these
- a)temperature
- a)Nematic Liquid Crystals
- b)Smectic Liquid Crystals
- c)Cholestric liquid Crystals
- a)High temperature
- d)Lyotropic Liquid Crystals
References:
1. GAURAV KUMAR JAIN – THEORY & PRACTICE OF PHYSICAL PHARMACY, 1st edition 2012 Elsevier, page no. 13-15.
2. Martins Physical Pharmcy, 6th edition 2011, page no. 56-77.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE