METHACHOLINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
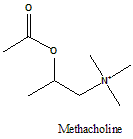
Methacholine
IUPAC nomenclature
2-(Acetyloxy)-N,N,N-trimethylpropan-1-aminium.
Classification
Methacholine is a cholinergic agonist falls in the class of choline esters.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 160.23 g/mol |
| 2 | Physical appearance | Present in solid form |
| 3 | Melting point | 171-173°C |
| 4 | Solubility | Water solubility is 0.0947mg/ml |
| 5 | Presence of ring | Not present |
| 6 | Number of chiral centers | 1 |
Mechanism of Action
- Methacholine induces bronchoconstriction by agonizing the muscarinic receptors.
- Therefore, this is used during the test of asthma and bronchial hyperreactivity.
Structure Activity Relationship
- When the nitrogen of the quaternary ammonium group is replaced with arsenic, phosphorous, sulfur or selenium, their activity decreases.
- It is necessary for the atom present at the nitrogen position to have a positive charge to have muscarinic activity.
- When all three methyl groups are replaced by larger alkyl groups at the quaternary nitrogen, drug becomes inactive as agonist.
- When all the methyl groups associated with the quaternary nitrogen atom are replaced by ethyl groups, the compound becomes cholinergic antagonist.
- Replacement of just one methyl group associated with quaternary nitrogen with an ethyl or a propyl group gives the compound which is having lesser activity than acetylcholine.
- Substitution of the methyl groups associated with quaternary nitrogen with hydrogen atoms also leads to diminishing of the muscarinic activity.
- At the ethylene bridge, synthesis of acetic acid esters of quaternary ammonium alcohols of greater length than choline led to a series of compounds which are having lesser activity as the length of the chain increases.
- There should be no more than 5 atoms between nitrogen and terminal hydrogen to have the maximum muscarinic activity.
- Replacement of the ethylene bridge by the alkyl groups larger than methyl groups decreases the activity.
- The R-(-) isomer is 20 time less potent.
- For the maximum potency, the alkyl groups at the nitrogen atoms should not be larger than methyl groups.
- Presence of oxygen in an ester form increases the activity.
- For maximum activity, there should be two- carbon atoms between the nitrogen and oxygen atom. [1]
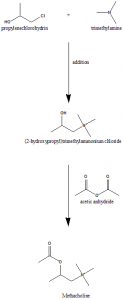
Method of synthesis
i. Addition of trimethylamine to propylene chlorohydrins gives (2-hydroxypropyl)trimethylammonium ion.
ii. The latter compound on reaction with acetic anhydride forms methacholine.
Therapeutic Uses
Methacholine is used for test of:
- Asthma
- Bronchial hyperreactivity
Side Effects
Side effects of methacholine are:
- Dizziness
- Nausea
- Vomiting
- Lightheadedness
- Sore throat
- Headache
MCQs
Q.1 Correct statements related with the Physicochemical properties of Methacholine are?
I. Molecular weight = 160.23 gm/mol
II. Physical appearance : present in viscous liquid form
III. Melting point = 35°C
IV. Solubility: very soluble in water
a) I, III
b) I, II, IV
c) II, IV
d) I
Q.2 Match the following of the drugs with their correct IUPAC names.
| i. Methacholine | A. 2-(Acetyloxy)-N,N,N-trimethylpropan-1-aminium. |
| ii. Homatropin | B. (RS)-(8-Methyl-8-azabicyclo[3.2.1]oct-3-yl) 3-hydroxy-2-phenylpropanoate |
| iii. Procyclidine | C. (N-Methyl-8-azabicyclo[3.2.1]oct-3-yl) 2-hydroxy-2-phenylacetate |
| iv. Atropin | D. 1-cyclohexyl-1-phenyl-3-pyrrolidin-1-ylpropan-1-ol |
a) i-B, ii-A, iii-D, iv-C
b) i-A, ii-C, iii-D, iv-B
c) i-D, ii-C, iii-B, iv-A
d) i-A, ii-B, iii-C, iv-D
Q.3 Correct steps for the mechanism of action of the drug Methacholine?
I. Antagonizing muscarinic receptors
II. Agonizing muscarinic receptors
III. Induction of bronchoconstriction
a) I – III
b) II – III
c) III – I
d) III – II
Q.4 Correct sequence for True/false for the classification of the drug can be?
- Methacoline: Cholinergic antagonist
- Procyclidine: Muscarinic antagonist
- Homatropin: Cholinergic agonist
- Atropin: Muscarinic antagonist
a) FTFT
b) TTTF
c) TFTF
d) FFTT
Q.5 Number of atoms requires between nitrogen and terminal hydrogen to have maximum activity for Methacholine is?
a) 1
b) 4
c) 5
d) 7
Q.6 The correct sequence for the steps for synthesis of drug Methacholine from Propyline chlorohydrine?
I. Reaction with trimethylamine
II. Reaction with acetic anhydride
III. Reduction
IV. Oxidation
a) I – II
b) I – III – II
c) I – III – II – IV
d) III – II – IV
Q.7 Side effect of drug Methacholine?
a) Dizziness
b) Sore throat
c) Lightheadedness
d) All of the above
FREE GPAT online Test: Participate: Click Here
ANSWERS
1-d
2-b
3-b
4-a
5-c
6-a
7-d
REFERENCES
[1] Lemke TL, Williams DA, Foye WO. Principles of medicinal chemistry. Williams & Wilkins; 2017, 320.