METHYCLOTHIAZIDE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Methyclothiazide
IUPAC nomenclature
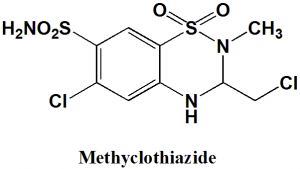
6-Chloro-3-(chloromethyl)-2-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide
Classification
- Thiazide diuretic
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 360.2 g/mol |
| 2 | Physical appearance | White crystalline powder |
| 3 | Melting point | 225oC |
| 4 | Solubility | Slightly soluble in alcohol; freely soluble in acetone |
| 5 | Octanol/water partition coefficient | 1.42 |
| 5 | Presence of ring | Benzothiazine |
| 6 | Number of chiral centers | Not present |
Mechanism of Action
- Methyclothiazide prevents active chloride reabsorption at the early distal tubule through the sodium chloride contransportor which results in an increase in the excretion of sodium, chloride and water from the body.
- The drug also binds with thiazide-sensitive Na-Cl transportor and prevents sodium ion transport across the renal tubular epithelium. This increases the potassium excretion through Na-K exchange mechanism.
- It can also mediate its actions on carbonic anhydrase in the smooth muscle or on the large-conductance KCa channel found in smooth muscle.
Structure Activity Relationship
General structure activity of thiazide diuretics can be summarized as:
- Chlorothiazide is the simplest member of the series.
- Hydrogen atom at the 2-N is most acidic due to presence of electron-withdrawing group.
- Sulfonamide group at C-7 position provides additional acidity to the drug.
- Electron withdrawing group is essential at position 6 for diuretic activity of the drug.
- Substitution oh hydrogen at 6 position gives little diuretic activity, whereas, substitution with chloro and trifluoromethyl groups gives highly active compounds.
- Substitution of electron donating group at position 6 significantly reduces the diuretic activity.
- Replacement or removal of sulfonamide groups from position 7 significantly reduces the diuretic activity.
- Saturation of the double bond to give 2,4-dihydro derivative are 10-folds more active than the unsaturated compounds.
- Substitution of a lipophillic group at 3 position increases the potency.
- Substitution with the entities such as haloalkyl, aralkyl or thioether gives compounds with longer duration of action due to increased lipid solubility.
- Alkyl substitution at the 2-N position can increase the action duration. [1]
Method of synthesis
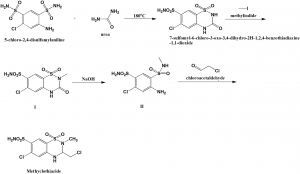
i. Cyclization of 5-chloro-2,4-disulfamoylaniline by using urea to get 7-sulfamoyl-6-chloro-3-oxo-3,4-dihydro-2H-1,2,4-benzothiadiazine-1,1-dioxide.
ii. Above formed compound is reacted with methyliodide in presence of sodiumhydride, taking DMF as solvent to get an intermediate compound (I).
(I) reacts with sodium hydroxide to give the compound (II).
(II) reacts with chloroacetaldehyde to get methyclothiazide.
Medicinal Uses
Methyclothiazide is used for:
- Management of hypertension
- Management of edema related to CHF, chronic renal failure, glomerulonephritis, nephritic syndrome and hepatic cirrhosis.
- To increase the effects of other antihypertensive drugs.
Side Effects
Side effects of methyclothiazide are:
- Nausea
- Blurred vision
- Vomiting
- Dizziness
- Fatigue
- Diarrhea
- Low blood sodium and potassium levels
- Loss of appetite
- Stomach cramps
- Sexual problems
- High blood sugar level
- Low blood pressure
- Muscle cramps
- Fainting
- Seizures
MCQs
Q.1 Mechanism of action of Methyclothiazide includes?
a) Prevention of active chloride reabsorption at the early distal tubule
b) Binding with thiazide sensitive Na-Cl transportor
c) None of these
d) Both a) and b)
Q.2 Therapeutic use of drug methyclothiazide is/are?
a) Management of diabetes
b) Treatment of Hypertension
c) Treatment of stable angina
d) All of the above
Q.3 Which of the following statement is correct related with the SAR of dihydropyridines calcium channel blockers?
a) Chlorothiazide is the simplest member of the series.
b) Hydrogen atom at the 2-N is most acidic due to presence of electron-withdrawing group.
c) Sulfonamide group at C-7 position provides additional acidity to the drug.
d) All of the above
Q.4 Number of chiral centers present in the structure of methyclothiazide?
a) 3
b) 2
c) 1
d) 0
Q.5 Correct sequence for the True/False for the physiochemical properties of the drug methyclothiazide can be?
I. Molecular weight = 246.3 gm/mol
II. Physical appearance = Colorless to yellowish liquid
III. Melting point = 173 oC
a) TFT
b) FFT
c) FFF
d) TTT
Q.6 Correct statements for the IUPAC nomenclatures of the drug are?
I. Methyclothiazide: 6-Chloro-3-(chloromethyl)-2-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide
II. Omeprazole: (RS)-6-(Difluoromethoxy)-2-[(3,4-dimethoxypyridin-2-yl)methylsulfinyl]-1H-benzo[d]imidazole
III. Methysergide: (RS)-1-[(4-chlorophenyl)- phenyl-methyl]-4- [(4-tert-butylphenyl) methyl] piperazine
IV. 5-FU: 1-cyano-2-methyl-3-[2-[(5-methyl-1H-imidazol-4-yl)methylsulfanyl]ethyl]guanidine
a) I, II, III, IV
b) III, IV
c) II, III, IV
d) I
Q.7 Match the following drugs with their correct classifications-
| i. Methyclothiazide | A. Diuretics |
| ii. Lansoprazole | B. Antimetabolite |
| iii. Thioguanine | C. Antihyperlipidemic |
| iv. Cholestipol | D. Proton pump inhibitor |
a) i-A, ii-C, iii-D, iv-B
b) i-A, ii-D, iii-B, iv-C
c) i-D, ii-B, iii-A, iv-C
d) i-D, ii-A, iii-C, iv-B
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
ANSWERS
1-d
2-b
3-d
4-d
5-c
6-d
7-b
REFERENCES
[1] Lemke TL, Williams DA, editors. Foye’s principles of medicinal chemistry. Lippincott Williams & Wilkins; 2012 Jan 24.
2] Close, W.J. et al.: J. Am. Chem. Soc. (JACSAT) 82, 1132 (1960).