MITOMYCIN C Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
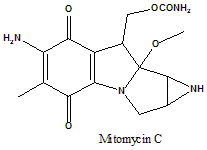
Mitomycin C
IUPAC nomenclature
{11-Amino-7-methoxy-12-methyl-10,13-dioxo-2,5-diazatetracyclo[7.4.0.02,7.04,6]trideca-1(9),11-dien-8-yl}methyl carbamate.
Classification
Mitomycin C falls under the category of antibiotic Antineoplastic cytotoxic drug. [1]
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 334.33 g/mol |
| 2 | Appearance | Blue-violet crystal form |
| 3 | Melting point | More than 360°C |
| 4 | Solubility | Soluble in water |
| 5 | Presence of ring | Aziridine ring |
Mechanism of Action
i. Mitomycin gets activated to bifunctional and trifunctional alkylating agent
ii. It binds with DNA and produces cross-linkings.
iii. DNA synthesis and DNA functions are inhibited and disturbed.[2]
Structural Activity Relationship
- Electron donating substituent increases the anticancer activities.
- The attached groups on N-1 positions are responsible for different biological activities of drug.
- Methylene bridge between the anthraquinone scaffold and aromatic ring can be neglected.
- When methylene groups are removed, substitution at ortho and para positions will increase the activity of drug.
- Counter ions of the anthraquinone are not responsible for anticancer activity.
- Strong electron donating groups can significantly increase the anticancer activity of drug. [3]
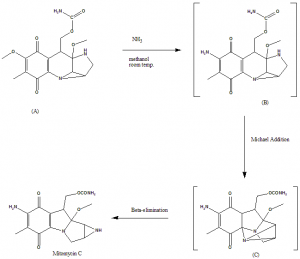
Methods of Synthesis
i. Compound (A) is treated with ammonia in methanol at room temperature to get the intermediate compound (B).
ii. (B) undergoes Michael addition to give an unstable compound (C).
iii. After Beta-elimination of the compound (C), Mitomycin C can be obtained.
Therapeutic Uses
Mitomycin is used for the treatment of:
- Adenocarcinoma
- Anal cancer
- Bladder cancer
- Breast cancer
- Cervical cancer
- Colorectal cancer
- Head and neck cancer
- Non-small cell lung cancer
Side Effects
- Common side effects include fatigue, low blood counts, poor appetite and mouth sores.
- Some people may suffer from side effects like bladder inflammation, loss of hair, diarrhea, nausea and vomiting.
MCQs
Q.1 Which amongst the following is the trade name of drug Mitomycin C?
a) Mutamycin
b) Novantrone
c) Blenoxane
d) None of these
Q.2 Predict the incorrect statement related to the therapeutic uses of drug Mitomycin C.
a) It is used for treatment of anal and bladder cancer.
b) It is not used for the treatment of pneumonia
c) It is used for the treatment of head and neck cancers
d) It is not used for the treatment of colorectal cancer
Q.3 Match the following with respect to the SAR of drug Mitomycin-
| i. Electron donating group substitution | A. Not responsible for anticancer activity |
| ii. Attached groups on N-1 position | B. Can be neglected |
| iii. Methylene bridge between anthquinone and aromatic ring | C. Increases the activity of drug |
| iv. Counter ions of anthraquinone | D. Responsible for biological activity of drug. |
a) i-C, ii-D, iii-B, iv-A
b) i-A, ii-C, iii-D, iv-B
c) i-B, ii-D, iii-A, iv-C
d) i-A, ii-D, iii-B, iv-C
Q.4 Which amongst the following drugs shows its effect through introduction of cross-linkages between the DNA strands?
a) Letrozole
b) Mitomycin C
c) Vincristine
d) Nafarelin
Q.5 Mitomycin C drug belongs to which class?
a) Antibiotic antineoplastic drug
b) Aromatase inhibitors
c) Alkylating agent
d) Both a) and c)
Q.6 Which of the following is not a side effect of Mitomycin C?
a) low blood counts
b) Poor appetite
c) Hair loss
d) None of the above
Q.7 Which amongst the following drugs is having least number of ring system in its structure-
a) Mitomycin C
b) Daunorubicin
c) Mitoxantrone
d) Bleomycin
ANSWERS
1-d
2-d
3-a
4-b
5-d
6-d
7-c
REFERENCES
[1] Tripathi KD. Essentials of Medical Pharmacology, 6thEdn. Jaypee Brothers Medical Publishers (P) Ltd. 2008: 820. [2] Verweij J, Pinedo HM. Mitomycin C: mechanism of action, usefulness and limitations. Anticancer Drugs. 1990 Oct 1;1(1):5-13. [3] Kunz KR, Iyengar BS, Dorr RT, Alberts DS, Remers WA. Structure activity relationship for mitomycin c and mitomycin a analogs. Journal of medicinal chemistry. 1991 Jul;34(7):2281-6.