NAFARELIN Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
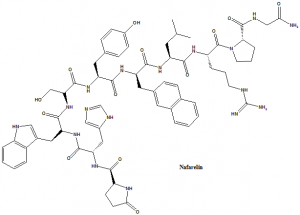
Nafarelin
IUPAC nomenclature
(2R)-N-[(2R)-5-carbamimidamido-1-[(2S)-2-[(carbamoylmethyl)carbamoyl]pyrrolidin-1-yl]-1-oxopentan-2-yl]-2-[(2R)-2-[(2R)-2-[(2R)-3-hydroxy-2-[(2S)-2-[(2S)-3-(1H-imidazol-4-yl)-2-{[(2R)-5-oxopyrrolidin-2-yl]formamido}propanamido]-3-(1H-indol-3-yl)propanamido]propanamido]-3-(4-hydroxyphenyl)propanamido]-3-(naphthalen-2-yl)propanamido]-4-methylpentanamide.
Classification
Nafarelin is a GnRH analogue. [1]
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 1322.5 g/mol |
| 2 | Appearance | White to off white solid |
| 3 | Melting point | More than 181°C |
| 4 | Solubility | Water solubility is 0.0166 mg/ml |
| 5 | Presence of ring | Ring structures present |
| 6 | Number of Chiral centers | 9 |
Mechanism of Action
i. Initial administration of Nafarelin stimulates the releases if Gonadotropins luteinizing hormone and follicle-stimulating hormone. From the pituitary gland. This results in the increase in the production of estradiol in females and testosterone in both the genders.
ii. When Nafarelin is administered continuously, it occupies the GnRH receptors leading to reversible down-regulation of the GnRH receptors in the pituitary and desensitization of the pituitary gonadotrophs.
iii. Due to this, there is significant decline in the production of FSH and LH.
iv. This causes decrease in the production of the estradiol, progesteron and testosterone by the ovaries or testes
v. Normal endomatrium and endomatrium implants contains estrogen receptors. Nafarelin induce anovulation and amenorrhea and decreases serum concentration of estradiol to the postmenaupausal range.
vi. This leads to atrophy of endomatriotic implants.
Structure Activity Relationship
- When Gly 6 is replaced by certain D-amino acids, or if there is change in the G-terminus, they became less susceptible to proteolytic enzymes, thus, provides longer lasting actions.
- Half life of the drug can be increased by substituting hydrophobic D-amino acids at the glycine near endopeptidase cleavage. [2]
Method of synthesis
I. Preparation of Boc-Gly-O-Resin:
i. 9 g of Nα-Boc-glycine was dissolved in a mixture of 50ml ethanol and 50 ml distilled water.
ii. pH of solution was brought to 7.5 using aqueous cesium bicarbonate.
iii. It was kept for drying for 18 hours under high vacuum.
iv. Residue was dissolved in 150ml dry DMF.
v. 25gm Merri-field resin was added.
vi. Mixture was shaken at 323K for 1 day and then was sequentially washed with DMF, water and ethanol.
vii. Resin was dried for three days under vacuum.
II. Synthesis of Nafarelin:
i. 1 mmol from preparation A was placed in the reaction vessel of a 5 L Vega 296 automatic solid phase peptide synthesizer.
ii. Following amino acids were added to the Preparation A resin:
- Nα-Boc-Pro 2 equiv.
- Nα-Boc-Arg 2 equiv.
- Nα-Boc-Leu 2 equiv.
- Nα-Boc-D-NaI 1.5 equiv.
- Nα-Boc-Tyr 1.5 quiv
- Nα-Boc-Ser(tBu) 2 equiv.
- Nα-Boc-Trp 1.75 equiv.
- Nα-Boc-His(tos) 1.75 equiv.
- (pyro)Glu 2.5 equiv.
iii. Crude peptide was dissolved in 2M acetic acid and converted to acetate salt by passage through a column of AG3-X4A resin.
iv. Acetate was dissolved in minimal amount of methanol.
v. It was then reprecipitated using acetone.
vi. It was then purified using HPLC to get the Nafarelin. [3]
Therapeutic Uses
The drug used for the treatment of:
- Endometriosis
- Precocious puberty
- Uterine fibroids
- Hirsutism
- Polycystic ovary syndrome
- Benign prostatic hyperplasia
Nafarelin is also used for
- Controlling ovarian stimulation in in vitro
- Puberty blocker in transgender
Side Effects
Side effects of Nafarelin includes
- hot flashes,
- vaginal drynesss,
- headaches,
- mood changes,
- erectile dysfunction,
- acne,
- muscle pain,
- reduced breast size,
- nasal irritation and some other sexual dysfunctions.
MCQs
Q.1 Brand name of Nafarelin is?
a) Synarel
b) Imuran
c) Transimune
d) Azoprine
Q.2 Which amongst the following statements is/are incorrect related to the SAR of Nafarelin?
I. When Gly 6 is replaced by certain D-amino acids, drug provides longer lasting actions
II. When Gly 6 is replaced by certain D-amino acids, drug become more susceptible to proteolytic activity.
III. Half life of the drug can be increased by substituting hydrophobic D-amino acids at the glycine near endopeptidase cleavage.
a) I only
b) II & III
c) II only
d) I, II & III
Q.3 Melting point of Nafarelin is?
a) More than 181°C
b) More than 250°C
c) Less than 150°C
d) 135.25°C
Q.4 The drug Nafarelin is mainly used for?
a) Treatment of Benign prostatic hyperplasia
b) As an anti-convulsive drug
c) Treatment of asthma
d) None of the above
Q.5 Match the drugs with the correct classification.
| i. Thiotepa | A. Purine antagonist |
| ii. Methotrexate | B. Folate antagonist |
| iii. Azathioprine | C. Ethylinimine |
| iv. Nafarelin | D. GnRH analogue |
a) i-B, ii-C, iii-A, iv-D
b) i-C, ii-A, iii-D, iv-B
c) i-B, ii-D, iii-C, iv-A
d) i-C, ii-B, iii-A, iv-D
Q.6 How many statements below are CORRECT with respect to the side effects of the drug Nafarelin?
- Hot flashes
- Erectile dysfunction
- Increase in the breast size
- Nasal irritation
a) 0
b) 1
c) 2
d) 3
Q.7 Number of chiral centers in the structure of Nafarelin is?
a) 5
b) 7
c) 9
d) 11
ANSWERS
1-a
2-c
3-a
4-a
5-d
6-b (breast size reduction is a side effect of nafarelin)
7-c
REFERENCES
[1] Tripathi KD. Essentials of Medical Pharmacology, 6thEdn. Jaypee Brothers Medical Publishers (P) Ltd. 2008: 820. [2] Lemke TL, Zito SW, Roche VF, Williams DA. Essentials of Foye’s principles of medicinal chemistry. Wolters Kluwer; 2017, 221. [3] Nestor Jr JJ, McClure NL, Arzeno H, inventors; Syntex USA LLC, assignee. Temporary minimal protection synthesis of serine-containing polypeptides. United States patent US 5,212,288. 1993 May 18.