NIMODIPINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
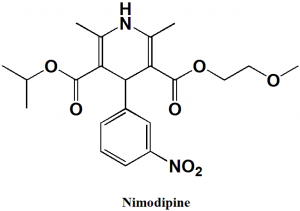
Nimodipine
IUPAC nomenclature
3-(2-Methoxyethyl) 5-propan-2-yl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
Classification
- Dihydropyridines
- Calcium channel blockers
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 418.4 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | 125oC |
| 4 | Solubility | 1.20e-02 gm/L |
| 5 | Octanol/water partition coefficient | 3.805 |
| 5 | Presence of ring | Dihydropyridine, phenyl |
| 6 | Number of chiral centers | 1 |
Mechanism of Action
i. Blocking of intracellular influence of calcium ions through voltage-dependent and receptor-operated slow calcium channels across the membrane of vascular smooth muscles and myocardial muscles.
ii. The drug specifically binds with L-type voltage gated calcium channel and inhibits the calcium ion transfer.
iii. Due to this, there is inhibition of vascular smooth muscle contraction.
iv. There is also direct inhibition of calcium overload in the neurons by the drug which helps in the treatment of subarachnoid hemorrhage.
Structure Activity Relationship
General structure activity of dihydropyridines calcium channel blockers can be summarized as:
- R1 should be unsubstituted.
- R1 should be easily detachable group.
- Basic amino ethyl ether chain at R2 increases the potency of drug, while H/aryl group results in loss of activity of drug.
- Substitution of R3 and R5 with alkoxy carbonyl group gives optimum activity.
- Branching of alkyl chain of ester group decreases the activity.
- R4 must be phenyl ring.
- S-enantiomers are more active than R-enantiomers.
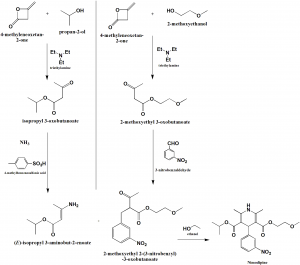
Method of synthesis
- Synthesis of (E)-isopropyl 3-aminobut-2-enoate:
- 4-methyleneoxetan-2-one reacts with propan-2-ol in presence of triethylamine to give isopropyl-3-oxoutanoate.
- Above formed compound is reacted with 4-methylbenzenesulfonic acid in presence of ammonia to give (E)-isopropyl 3-aminobut-2-enoate.
- Synthesis of 2-methoxyethyl 2-(3-nitrobenzyl)-3-oxobutanoate:
- 4-methyleneoxetan-2-on is reacted with 2-methoxyethanol in presence of triethylamine to give 2-methoxyethyl 3-oxobutanoate.
- Above formed compound is reacted with 3-nitrobenzaldehyde to give 2-methoxyethyl 2-(3-nitrobenzyl)-3-oxobutanoate
- Synthesis of nimodipine:
Final compound formed in steps I and II are reacted together in the presence of ethanol to give nimodipine.
Medicinal Uses
Nimodipine is used for:
- As an adjunct to improve the neurologic outcome following SAH (subarachnoid hemorrhage)
Side Effects
Side effects of Nimodipine are:
- Dizziness
- Weakness
- Flushing
- Fainting
- Lightheadedness
- Swelling of ankles
- Headache
- Abdominal pain
- Nausea
- Vomiting
- Trouble breathing
- Allergic reactions
MCQs
Q.1 Match the following with correct SAR of the dihydropyridines calcium channel blockers –
| i. S-enantiomers are | A. More active than R-enantiomers |
| ii. Branching of alkyl group of ester chain | B. Less active than R-enantiomers |
| C. Increases the activity | |
| D. Decreases the activity |
a) i-A, ii-C
b) i-A, ii-D
c) i-B, ii-C
d) i-B, ii-D
Q.2 Correct sequence for the True/False for correct IUPAC names of the drug can be?
- Nimodipine: 3-(2-Methoxyethyl) 5-propan-2-yl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
- Naproxen: [2-(2,6-Dichloroanilino)phenyl]acetic acid
- Chlorpromazine: (−)-17-allylmorphinan-3-ol
- Sodium salicylate: Sodium;2-hydroxybenzoate
a) FFTF
b) FFFF
c) TFFT
d) TTTT
Q.3 Molecular weight of drug Nimodipine is?
a) 227.09 gm/mol
b) 652.36 gm/mol
c) 142.45 gm/mol
d) 418.4 gm/mol
Q.4 Mechanism of action of nimodipine includes?
a) Inhibition of calcium overload in the neurons
b) Conversion into nitric oxide
c) Inhibition of hydrogen-potassium pump
d) All of the above
Q.5 Which amongst the following is a therapeutic use of drug nimodipine?
a) Treatment of angina
b) Improving the neurologic outcome following SAH
c) As an antidiabetic drug
d) As an anesthetic
Q.6 Which of the following drug and their classification are correct?
I. Nimodipine: Calcium channel blocker
II. Neostigmine: Cholinestrase inhibitor
III. Felbamate: Antineoplastic drug
IV. Mechlorethamine: Antineoplastic drug
a) I ,II, IV
b) II, IV
c) I, II, III, IV
d) I, III
Q.7 Type of ring present in the structure of nimodipine?
a) Dihydrofuran
b) Dihydropyridine
c) Pyrimidine
d) Pyridine
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
ANSWERS
1-b
2-c
3-d
4-a
5-b
6-a
7-a
REFERENCES
[1] Meyer, H. et al.: Arzneim.-Forsch. (ARZNAD) 31, 407 (1981); 33, 106 (1983).