Oxygen flask combustion: Apparatus, Method with Diagram; Question, Answers and Explanation
Oxygen flask combustion :-
Wolfgang schoniger is the chemist who first proposed this method in 1955.
Oxygen flask combustion method has been used to determine of sulfur & Halogen in medicine.
Apparatus :-
The apparatus consists of heavy walled conical , deeply liped or cupped 500 ml flask fitted with a ground glass stopper to which is fused a test specimen carrier consisting gague platinum wire.
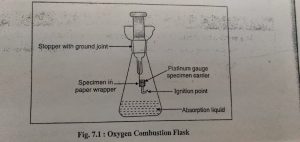
If the ignition is carried out in a closed system, the inversion of flask may be omitted.
After combustion is complete shake the flask vigorously and allow to stand for not less than 10 minute with intermittent shaking. Then proceed as directed for particular ion.
For iodine :-
10 ml water + 2 ml 1N NaoH as absorbing liquid
⇓
Add 5-10 ml of acetic bromine solution.
⇓
Remove excess bromine by addition of 1 ml formic acid + remove bromine vapor with current of air + 1 gm KI
⇓
Titrate with 0.02N Na2S2O3 using starch solution as indicator
Factor :- Each ml of 0.02M Na2S2O3 is equivalent to 0.000423 gm of Iodine.
For Sulphur :-
Method 1 ( In absence of Halogen and phosphorus )
Absorbing :- 10 ml of water + 0.1 ml of H2O2 (30%)
⇓
Cool the solution in ice for 15 min boil for 2 minutes + 50 ml of ethanolic acetic ammonia buffer (pH =3.7)
⇓
Titrate with 0.05 M barium perchlorate using 0.3 ml alizarin red S as indicator until solution become orange pink in colour.
Factor :- Each ml of 0.05 M barium perchlorate is equivalent to 0.001603 gm of S.
MCQ
1. Which method are used to determination of sulfur and Halogen in medicine ?
A. Karl-fischer titration
B. Diazotization titration
C. Oxygen Flask combustion
D. All of the above
2. Which type of filter paper are used in oxygen Flask combustion ?
A. What man filter Paper
B. Halide free filter paper
C. A and B
D. None of the above
3. How many ml of liquid filled in polycarbonate capsule ?
A. 200 micro litre
B. 100 micro litre
C. 100 litre
D. 200 litre
4. How many ml flask are used in oxygen Flask combustion ?
A. 500 ml
B. 400 ml
C. 199 ml
D. 300 ml
5. Which material are used in gauge ?
A. Platinum
B. Iron
C. Copper
D. Aluminum
Answer key
1. C
2. B
3. A
4. A
5. A
Participate in Online FREE GPAT TEST: CLICK HERE