PANTOPRAZOLE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Pantoprazole
IUPAC nomenclature
(RS)-6-(Difluoromethoxy)-2-[(3,4-dimethoxypyridin-2-yl)methylsulfinyl]-1H-benzo[d]imidazole
Classification
- Proton pump inhibitors
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 383.4 g/mol |
| 2 | Physical appearance | Off-white solid |
| 3 | Melting point | 139-140oC |
| 4 | Solubility | Freely soluble in water |
| 5 | Octanol/water partition coefficient | 2.05 |
| 5 | Presence of ring | Benzimidazole, pyridine |
| 6 | Number of chiral centers | Not present |
Mechanism of Action
- Pantoprazole is a prodrug which requires protonation via an acidic environment to get activated form Sulphenamide.
- Sulphenamide covalently binds with cystien residues via disulfide bridges on the α subunit of H/K ATPaseenzyme system.
- Thereby it inhibits the H+/K+ ATPase pump which in turn inhibits the gastric acid secretion or formation of hydrochloric acid in parietal cells
Structure Activity Relationship
General structure activity of Heteroaryl- and heterocyclyl-substituted imidazo[1,2-a]Pyridine derivatives acting as acid pump antagonists can be summarized as:
- The inhibitory property of drugs depends on hydrophobic group substituted at the left side ortho-position of the phenyl ring.
- Increase in hydrophobic value increases the activity.
- Increasing the GTCI value decreases the activity.
- Small molecule substitutions may participate in hydrophobic interaction as well as steric interactions. [1]
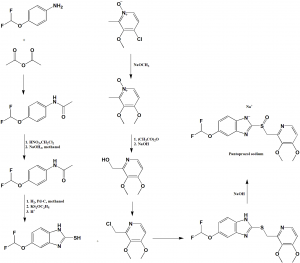
Method of synthesis
I. Synthesis of 5-(difluoromethoxy)-2-mercaptobenzimidazole:
i. 4-(difluoromethoxy)-aniline is reacted with acetic anhydride to get N-(4-(difluoromethoxy)phenyl)acetamide.
ii. It is then treated with HNO3 followed by reaction with NaOH3 in methanol to get N-(4 (difluoromethoxy)phenyl)acetamide.
iii. After hydrogenation and acidification, the desired compound is formed.
II. Synthesis of 2-chloromethyl-3,4-dimethoxy-pyridinium chloride:
i. 4-chloro-3-methoxy-2-methylpyridine-N-oxide reacted with NaOCH3 followed by reaction with acetic anhydride and treatment with sodium hydroxide followed by chlorination to get the desired compound.
III. Synthesis of Pantoprazole:
i. Both the compounds formed in I and II are reacted together to get pantoprazole. [2]
Medicinal Uses
Pantoprazole is used for treatment of:
- Stomach ulcers
- Active duodenal ulcers
- Active benign gastric ulcer
- GERD
- Erosive esophagitis
- Zollinger-Errison syndrome
- pylori eradication
Side Effects
Side effects of pantoprazole are:
- Abdominal pain
- Nausea
- Constipation
- Headache
- Low magnesium blood level
- Irregular heartbeat
- Muscle spasms
- Seizures
- Lupus
- Diarrhea
- Blood in stool
MCQs
Q.1 What can be the correct IUPAC nomenclature of Pantoprazole?
a) 2-(diphenylmethoxy)-N,N-dimethylethanamine
b) (RS)-6-(Difluoromethoxy)-2-[(3,4-dimethoxypyridin-2-yl)methylsulfinyl]-1H-benzo[d]imidazole
c) (RS)-1-[(4-chlorophenyl)- phenyl-methyl]-4- [(4-tert-butylphenyl) methyl] piperazine
d) (R)-(+)-2-([3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methylsulfinyl)-imidazole
Q.2 Which amongst the following statements is/are incorrect related to the General structure activity of Heteroaryl- and heterocyclyl-substituted imidazo[1,2-a]Pyridine derivatives acting as acid pump antagonists?
I. Increasing the GTCI value decreases the activity.
II. Small molecule substitutions may participate in hydrophobic interaction as well as steric interactions.
a) I
b) I, II
c) II
d) None
Q.3 Types of rings present in the structure of PANTOPRAZOLE?
I. Furan
II. Pyridine
III. Benzimidazole
IV. Phenyl
a) I, II
b) I, IV
c) II, IV
d) II, III
Q.4 Side effects of drug PANTOPRAZOLE is/are?
a) Abdominal pain
b) Constipation
c) Muscle spasms
d) All of the above
Q.5 Match the following drugs with their correct molecular weight-
| i. Pantoprazole | A. 166.24 gm/mol |
| ii. Chlorcyclizine | B.240.34 gm/mol |
| iii. Edrophonium | C. 383.4 gm/mol |
| iv. Pheniramine | D. 300.8 gm/mol |
a) i-A, ii-B, iii-C, iv-D
b) i-C, ii-A, iii-B, iv-D
c) i-C, ii-D, iii-A, iv-B
d) i-A, ii-C, iii-D, iv-B
Q.6 An example of drug from class Proton pump inhibitor drug?
a) Norepinephrine
b) Tolazoline
c) Pantaprazole
d) Phenylephrine
Q.7 Octanol/water partition coefficient of pantoprazole is?
a) 2.05
b) 3.2
c) 4.6
d) 1.6
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
ANSWERS
1-b
2-d
3-d
4-d
5-c
6-c
7-a
REFERENCES
[1] Agarwal N, Bajpai A, Gupta SP. A quantitative structure-activity relationship and molecular modeling study on a series of heteroaryl-and heterocyclyl-substituted imidazo [1, 2-a] pyridine derivatives acting as acid pump antagonists. Biochemistry Research International. 2013 Jan 1;2013.[2] [2] Vardanyan R, Hruby V. Synthesis of best-seller drugs. Academic press; 2016 Jan 7.