PHYSOSTIGMINE Synthesis, SAR, MCQ, Structure, Chemical Properties and Therapeutic Uses
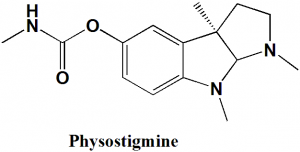
Physostigmine
IUPAC nomenclature
[(3aR,8bS)-3,4,8b-trimethyl-2,3a-dihydro-1H-pyrrolo[2,3-b]indol-7-yl] N-methylcarbamate.Classification
- Reversible anticholinestrase
- Carbamates
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 275.35 g/mol |
| 2 | Physical appearance | White microcrystalline powder |
| 3 | Melting point | 105.5oC |
| 4 | Solubility | Slightly soluble in water; very soluble in dichloromethane |
| 5 | Octanol/water partition coefficient | 1.58 |
| 6 | Presence of ring | Pyrrolo-pyrrole |
| 7 | Number of chiral centers | 2 |
Mechanism of Action
- Physostigmine increases the availability of the acetylcholine at the synapse by inhibiting acetylcholinestrase enzyme.
- Acetylcholinestrase enzyme is responsible for the metabolism of acetylcholine, and thus, inhibition of acetylcholinestrase indirectly stimulates nicotinic and muscarinic receptors.
Structure Activity Relationship
General SAR for carbamates anticholinestrases can be summarized as follows:
- Variation of the chain length influence AchE inhibition.
- Most potent AChE inhibitor for the series was found to be having 7 methyl groups attached in the chain.
- Replacement of the xanthone nucleus with other aryl groups decreases the AChE inhibitory activity.
- Presence of polar group on the aromatic moiety is essential for the activity of drug.
- If the flexible chain is replaced by the rigid linear ethyne group, activity of the drug is lost, which shows that, a flexible chain is necessary for the activity.
- Introduction of the tertiary amino group into saturated ring decreases the activity, but activity is not fully lost.
- Changing the length of the carbamic N-substituent has a significant effect on the selectivity of the drug. [1]
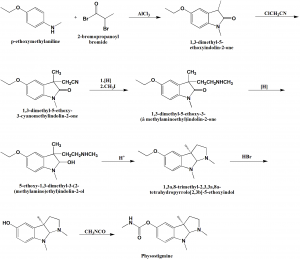
Method of synthesis
i. p-ethoxymethylaniline is reacted with α-bromopropionyl bromide in the presence of aluminum chloride to give 1,3-dimethyl-5-ethoxyindolin-2-one.
ii. The above formed compound is reacted with chloroacetonitrile in the presence of sodium ethoxide to give 1,3-dimethyl-5-ethoxy-3-canomethylindolin-2-one.
iii. On resucing the amine group which is later methoxided produces 1,3-dimethyl-5ethoxy-3-(ß-methylaminoethyl)indolin-2-one.
iv. Reduction of the carbonyl group to give an aminoalcohol
v. On dehydration of the aminoalcohol, 1,3a,8-trimethyl-2,3,3a,8a-tetrahydrophyrrolo[2,3b]-5-ethoxyindol is produced.
vi. By the help of hydrogen bromide, the ethoxyprotecting group is removed to give a compound with a phenolhydroxyl group.
vii. On reaction of the above formed compound with methylisocyanate yields physostigmine.[2]
Medicinal Uses
Physostigmine is used for:
- Treatment of glaucoma
- Treatment of severe anticholinergic toxicity
- For diagnosis purpose
Side Effects
Side effects of physostigmine are:
- Vomiting
- Diarrhea
- Abdominal cramps
- Diaphoresis
- Seizures
- Bradysystole
MCQs
Q.1 Match the following with correct SAR of the class Carbamates cholinestrases-
| i. Replacement of the xanthone nucleus with other aryl groups | A. Increases the AChE activity |
| ii. Replacement of the flexible chain f the drug with rigid alkyne chain | B. Decreases the AChE activity |
| C. leads to loss of activity | |
| D. leads to a compound with higher AChE activity |
a) i-A, ii-C
b) i-A, ii-D
c) i-B, ii-C
d) i-B, ii-D
Q.2 Correct sequence for the True/False for correct IUPAC names of the drug can be?
- Physostigmine: [(3aR,8bS)-3,4,8b-trimethyl-2,3a-dihydro-1H-pyrrolo[2,3-b]indol-7-yl] N-methylcarbamate
- Aurothioglucose: gold(1+);(3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxane-2-thiolate
- Nalorphine: 17-allyl-7,8-didehydro-4,5α-epoxymorphinan-3,6α-diol.
- Diclofenac: [2-(2,6-Dichloroanilino)phenyl]acetic acid
- a) TFTF
b) FFFF
c) TTTT
d) TFFT
Q.3 Molecular weight of drug Physostigmine is?
a) 275.35 gm/mol
b) 154.28 gm/mol
c) 406.21 gm/mol
d) 162.1 gm/mol
Q.4 Physostigmine mechanism of action includes?
a) It inhibits Acetylcholinestrase enzyme
b) The level of acetylcholine at the synapse decreases
c) It exerts effect on COX-1 and on COX-2
d) All of the above
Q.5 Which amongst the following is a therapeutic use of drug Physostigmine?
a) Treatment of Glaucoma
b) Reducing pain due to arthritis
c) Controlling vomiting
d) All of the above
Q.6 Which of the following drug and their classification are correct?
I. Physostigmine: NSAID
II. Vinblastin: Propionic acid derivative
III. Enflurane: Inhalational anesthetics
IV. Alprazolem: Benzodiazepine sedative-hypnotic
a) III, IV
b) I, II, III
c) I, II, III, IV
d) II
Q.7 Number of chiral carbons resent in the structure of Physostigmine is?
a) 4
b) 2
c) 1
d) 0
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-d
2-c
3-c
4-a
5-a
6-a
7-b