PILOCARPINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Pilocarpine
IUPAC nomenclature
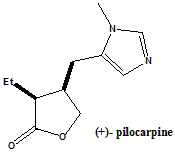
(3S,4R)-3-Ethyl-4-((1-methyl-1H-imidazol-5-yl)methyl)dihydrofuran-2(3H)-one.
Classification
Pilocarpine is a cholinergic agonist falls in the class of alkaloids.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 208.26 g/mol |
| 2 | Physical appearance | Present in oil or crystal (needle) form. |
| 3 | Melting point | 34°C |
| 4 | Solubility | Soluble in water, alcohol, chloroform; insoluble in petroleum ether. |
| 5 | Presence of ring | Imidazole and furane |
| 6 | Number of chiral centers | 2 |
Mechanism of Action
- Pilocarpine is a cholinergic parasympathomimetic agent.
- It mainly stimulates the muscarinic receptors.
- Thus, it increases secretion by the exocrine glands and produces contraction of the iris, sphincture muscle and ciliary muscle.
Structure Activity Relationship
- When the nitrogen of the quaternary ammonium group is replaced with arsenic, phosphorous, sulfur or selenium, their activity decreases.
- It is necessary for the atom present at the nitrogen position to have a positive charge to have muscarinic activity.
- When all three methyl groups are replaced by larger alkyl groups at the quaternary nitrogen, drug becomes inactive as agonist.
- When all the methyl groups associated with the quaternary nitrogen atom are replaced by ethyl groups, the compound becomes cholinergic antagonist.
- Replacement of just one methyl group associated with quaternary nitrogen with an ethyl or a propyl group gives the compound which is having lesser activity than acetylcholine.
- Substitution of the methyl groups associated with quaternary nitrogen with hydrogen atoms also leads to diminishing of the muscarinic activity.
- At the ethylene bridge, synthesis of acetic acid esters of quaternary ammonium alcohols of greater length than choline led to a series of compounds which are having lesser activity as the length of the chain increases.
- There should be no more than 5 atoms between nitrogen and terminal hydrogen to have the maximum muscarinic activity.
- Replacement of the ethylene bridge by the alkyl groups larger than methyl groups decreases the activity.
- The R-(-) isomer is 20 time less potent.
- For the maximum potency, the alkyl groups at the nitrogen atoms should not be larger than methyl groups.
- Presence of oxygen in an ester form increases the activity.
- For maximum activity, there should be two- carbon atoms between the nitrogen and oxygen atom. [1]
Method of synthesis
- (+)-pilocarapine can be synthesized from racemic 2-(2’,2’-dimethoxyethyl)propane-1,3-diol.
- Firststep is the desymmetrization of the compound.
- Other steps involves the palladium-catalysed decarboxylation/carbonylation of a 1,3-dioxan-2-one for the formation of the γ-butyrolactone ring.
- It is followed by hydrogenation of the α-ethylidene lactone to give a mixture of pilocarpine and isopilocarpine.
- (+)-pilocarpine then can be separated through recrystallisation to give 30% overall yield. [2]
Therapeutic Uses
Pilocarpine is used for treatment of:
- Symptoms of dry mouth
- Aqueous deficient dry eye (ADDE)
Side Effects
Side effects of Pilocarpine are:
- Vision problems
- Diarrhea
- Weakness
- Dizziness
- Frequent maturation
- Flushing
- Chills
- Runny nose
- Nausea
- Sweating
MCQs
Q.1 Pilocarpine?
a) Stimulate nicotinic receptors
b) Stimulates muscarinic receptors
c) Inhibits nicotinic receptors
d) Inhibits muscarinic receptors
Q.2 Therapeutic use of drug Pilocarpine is/are?
a) Dry mouth
b) Dry eyes
c) Glaucoma
d) Both a) and b)
Q.3 Which amongst the following are the correct statements with respect to the SAR of drug pilocarpine.
I. For the maximum potency, the alkyl groups at the nitrogen atoms should not be larger than methyl groups.
I. Presence of oxygen in an ester form decreases the activity.
III. For maximum activity, there should be two- carbon atoms between the nitrogen and oxygen atom.
a) II
b) I
c) II, I
d) I, III
Q.4 The starting chemicals required for the synthesis of drug pilocarpine?
a) 2-(2’,2’-dimethoxyethyl)propane-1,3-diol.
b) Epoxypropane
c) Cyclobutane
d) Chlorhexane
Q.5 Correct sequence for the True/False for the physiochemical properties of the drug pilocarpine
I. Imidazole ring is present
II. Soluble in water
III. Present in oil or crystal form
a) TTT
b) TTF
c) FFT
d) FTF
Q.6 Correct statements for the IUPAC nomenclatures of the drugs are?
I. Pilocarpine: (3S,4R)-3-Ethyl-4-((1-methyl-1H-imidazol-5-yl)methyl)dihydrofuran-2(3H)-one.
II. Orphinendrine: N,N-dimethyl-2-[(2-methylphenyl)-phenylmethoxy]ethanamine;2-hydroxypropane-1,2,3-tricarboxylic acid.
III. Tiotropium: 7-[(hydroxidi-2-thienylacetyl)oxy]-9,9-dimethyl-3-oxa-9-azoniatricyclo[3.3.1.02,4]nonane bromide
IV. Carbachol: 2-[(Aminocarbonyl)oxy]-N,N,N-trimethylethanaminium chloride.
a) I, III
b) II, IV
c) I, II, III, IV
d) I, II, IV
Q.7 Match the following drugs with their correct classifications-
| i. Pilocarpine | A. Acetylcholinestrase inhibitor |
| ii. Physostigmine | B. Acetylcholine antagonist-muscarinic antagonist |
| iii. Orphenadrine | C. Acetylcholine mimetic-muscarinic agonist |
| iv. Metocurine | D. Nicotinic antagonist |
a) i-C, ii-A, iii-B, iv-D
b) i-B, ii-D, iii-A, iv-C
c) i-A, ii-B, iii-D, iv-C
d) i-B, ii-C, iii-D, iv-A
FREE GPAT online Test: Participate: Click Here
ANSWERS
1-b
2-d
3-d
4-a
5-a
6-c
7-a
REFERENCES
[1] Lemke TL, Williams DA, Foye WO. Principles of medicinal chemistry. Williams & Wilkins; 2017, 320. [2] Davies SG, Roberts PM, Stephenson PT, Storr HR, Thomson JE. A practical and scaleable total synthesis of the jaborandi alkaloid (+)-pilocarpine. Tetrahedron. 2009 Sep 26;65(39):8283-96.