PROCHLORPERAZINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
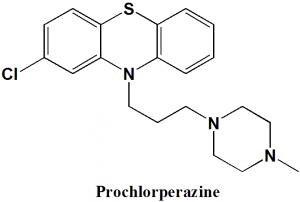
Prochlorperazine
IUPAC nomenclature
2-chloro-10-[3-(4-methyl-1-piperazinyl)propyl]-10H-phenothiazine
Classification
Prochlorperazine is phenothiazine antipsychotic drug.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 373.9 g/mol |
| 2 | Physical appearance | Viscous liquid |
| 3 | Melting point | 228°C |
| 4 | Solubility | Freely soluble in alcohol, ether, chloroform |
| 5 | Octanol/water partition coefficient | 4.88 |
| 6 | Presence of ring | Phenothiazine, piperazine |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
- Prochlorperazine blocks D2 dopamine receptors in brain. This results in blockade of postsynaptic dopamine receptors in the mesolimbic system and thus, and increased dopamine turnover.
- Drug shows antiemetic effects. It inhibits apomorphine-induced vomiting by blocking D2 dopamine receptors in the chemoreceptor trigger zone.
Structure Activity Relationship
Structure activity relationship of phenothiazine can be described as follows:
- Tilting of side chain towards ring A grants favorable Vander Waal’s interaction of the side chain. This interaction decides the potency of the drug towards the dopamine receptors.
- Optimal neuroleptic activity occurs when the ring A substituent is in the 2nd-position.
- A trifluoromethyl substituent provides a greater number of favorable Van der Waal’s contacts with the side chain than the chlorine substituent. Thus, phenothiazne with trifluoromethyl substituents are more potent than those with chlorine substituent.
- A piperazine side chain provides more Van der Waal’s contacts with 2-substituent than the alkylamino side chain. Thus, piperizine phenothiazine are more potent in antischizophrenic effects than alkylamino phenothiazines.
- Hydroxyethylpiperazine side chain phenothiazines displays more favorable Van der Waal’s interactions with ring A than simple piperazines.
- In the thioxanthene and xanthenes containing ring systems, the cis forms are more potent neuroleptics than the trans isomers.
- Phenothiazine analogues having the presence of exolytic double bond are more potent than the corresponding compounds lacking the exolytic double bonds. [1]
Method of synthesis
Prochlorperazine can be synthesized by the reaction of 2-chloro-10H-phenothiazine and 1-(3-chloropropyl)-4-methylpiperazine in the presence of sodium amide.
Therapeutic Uses
Prochlorperazine is used for:
- Treatment of nausea
- Treatment of vomiting
Side Effects
Side effects of Prochlorperazine are:
- Dizziness
- Drowsiness
- Lightheadedness
- Dry mouth
- Blurred vision
- Constipation
- Mood changes
- Agitation
- Restlessness
- Tremors
- Uncontrolled movements
- Neck twisting
- Enlarged breast
- Unusual production of milk
- Weakness
- Infection
- Abdominal pain
- Sore throat
MCQs
Q.1. The antiemetic effect of prochlorperazine is due to?
a) Blocking of D1 dopaminergic receptors
b) Blocking of D2 dopaminergic receptors
c) Blocking of α-adrenoceptors
d) Blocking of ß-adrenoceptors
Q.2 Therapeutic use of drug prochlorperazine is/are?
a) Treatment of nausea and vomiting
b) Treatment of cancer
c) Treatment of Parkinson’s disease
d) All of the above
Q.3 Which amongst the following are the correct statements with respect to the SAR of drug prochlorperazine?
I. Minimal neuroleptic activity occurs when the ring A substituent is in the 2nd-position.
II. A trifluoromethyl substituent provides a greater number of favorable Van der Waal’s contacts with the side chain than the chlorine substituent. Thus, phenothiazne with trifluoromethyl substituents are more potent than those with chlorine substituent.
III. A piperazine side chain provides more Van der Waal’s contacts with 2-substituent than the alkylamino side chain. Thus, piperizine phenothiazine are more potent in antischizophrenic effects than alkylamino phenothiazines.
IV. Hydroxyethylpiperazine side chain phenothiazines displays less favorable Van der Waal’s interactions with ring A than simple piperazines.
a) I, III, IV
b) II, IV
c) II, III
d) III, IV
Q.4 The starting chemicals required for the synthesis of drug prochlorperazine?
a) 2-chloro-10H-phenothiazine
b) 1-(3-chloropropyl)-4-methylpiperazine
c) Sodium amide
d) All of the above
Q.5 Correct sequence for the True/False for the physiochemical properties of the drug prochlorperazine?
- Molecular weight is 450 gm/mol
- It is present in viscous liquid form
- It is insoluble in alcohol
a) FTF
b) FFT
c) FTT
d) TFF
Q.6 Correct statements for the IUPAC nomenclatures of the are?
I. Prochlorperazine: 2-chloro-10-[3-(4-methyl-1-piperazinyl)propyl]-10H-phenothiazine
II. Phenytoin: 5-butan-2-yl-5-ethyl-1,3-diazinane-2,4,6-trione
III. Chloraquine: 5,5-diphenylimidazolidine-2,4-dione
IV. Favipiravir: 6-fluoro-3-hydroxypyrazine-2-carboxamide
a) I, II
b) I, IV
c) II, III
d) III, IV
Q.7 Match the following drugs with their correct classifications-
| i. Prochlorperazine | A. Nonbenzodiazepine sedative hypnotics |
| ii. Propantheline | B. Antimuscarinic bronchodilator |
| iii. Eszopiclone | C. Acetylcholine antagonist |
| iv. Trotopium | D. Phenothiazine antipsychotic drug |
a) i-C, ii-A, iii-D, iv-B
b) i-A, ii-C, iii-D, iv-B
c) i-B, ii-C, iii-D, iv-A
d) i-D, ii-C, iii-A, iv-B
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-B
2-A
3-C
4-D
5-C
6-A
7-D