PROMAZINE HYDROCHLORIDE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
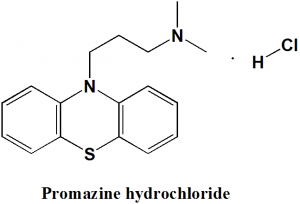
Promazine hydrochloride
IUPAC nomenclature
N,N-dimethyl-3-phenothiazin-10-ylpropan-1-amine;hydrochloride.
Classification
- Phenothiazine
- Antipsychotic
- Antiemetic
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 320.9 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | 174-176°C |
| 4 | Solubility | >48.1 ug/ml |
| 5 | Octanol/water partition coefficient | logP = 2.94 |
| 6 | Presence of ring | Phenothiazine |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
The post synaptic dopamine receptors D1 and D2 in the mesolimbic and medullary chemoreceptor trigger zone is blocked by Promazine hydrochloride. This results in decrease in stimulation of the vomiting center in the brain and psychotic effects including hallucinations and delusions.
Promazine hydrochloride also blocks α-adrenergic receptors and thus exhibit strong anticholinergic activity.
Structure Activity Relationship
Structure activity relationship of phenothiazine can be described as follows:
- Tilting of side chain towards ring A grants favorable Vander Waal’s interaction of the side chain. This interaction decides the potency of the drug towards the dopamine receptors.
- Optimal neuroleptic activity occurs when the ring A substituents is in the 2nd-position.
- A trifluoromethyl substituent provides a greater number of favorable Van der Waal’s contacts with the side chain than the chlorine substituent. Thus, phenothiazne with trifluoromethyl substituents are more potent than those with chlorine substituents.
- A piperazine side chain provides more Van der Waal’s contacts with 2-substituent than the alkylamino side chain. Thus, piperizine phenothiazine are more potent in antischizophrenic effects than alkylamino phenothiazines.
- Hydroxyethylpiperazine side chain phenothiazines display more favorable Van der Waal’s interactions with ring A than simple piperazines.
- In the thioxanthene and xanthenes containing ring systems, the cis forms are more potent neuroleptics than the trans isomers.
- Phenothiazine analogues having the presence of exolytic double bond are more potent than the corresponding compounds lacking the exolytic double bonds. [1]
Method of synthesis
i. Diphenylamine reacts with sulfur to give phenothiazine.
ii. Promazine can be synthesized from phenothiazine by alkylation with 3-dimethylaminopropylchloride in the presence of sodium amide. [2]
Therapeutic Uses
Promazine hydrochloride is used for:
- Treatment of Schizophrenia
Side Effects
Side effects of Promazine hydrochloride are:
- Anticholinergic side effects
- Extrapyramidal side effects
MCQs
Q.1 “N,N-dimethyl-3-phenothiazin-10-ylpropan-1-amine” is the IUPAC nomenclature of which drug?
a) Promazine
b) Chlorpromazine
c) Chlordiazepoxide
d) Phenobarbiton
Q.2 The approximate molecular weight of the drug promazine hydrochloride is?
a) 320 gm/mol
b) 222 gm/mol
c) 545 gm/mol
d) 465 gm/mol
Q.3 Match the following with correct classifications of the drugs.
| i. Promazine | A. 5-HT receptor antagonist |
| ii. Lysergic acid diethylamide | B. Hallucinogenic agent |
| iii. Alosetron | C. 5-HT receptor agonist |
| iv. Eletriptan | D. Phenothiazine |
a) i-B, ii-A, iii-C, iv-D
b) i-D, ii-A, iii-B, iv-C
c) i-A, ii-B, iii-D, iv-C
d) i-D, ii-B, iii-A, iv-C
Q.4 Type of receptors with which promazine interacts?
I. Dopamine D1 receptors
II. Dopamine D2 receptos
III. α-adrenoceptors
IV. ß-adrenoceptor
a) I, II
b) III, IV
c) I, II, III
d) II, III, IV
Q.5 Correct sequence for True and False for the given statements related with the SAR of drug promazine hydrochloride?
- Tilting of side chain towards ring A grants favorable Vander Waal’s interaction of the side chain. This interaction decides the potency of the drug towards the dopamine receptors.
- Optimal neuroleptic activity occurs when the ring A substituents is in the 2nd-position.
- A trifluoromethyl substituent provides a greater number of favourable Van der Waal’s contacts with the side chain than the chlorine substituent. Thus, phenothiazne with trifluoromethyl substituents are therefore less potent than those with chlorine substituents.
- A piperazine side chain provides lesser Van der Waal’s contacts with 2-substituent than the alkylamino side chain. Thus, piperizine phenothiazine are more potent in antischizophrenic effects than alkylamino phenothiazines.
a) TFTT
b) TTFF
c) FFTT
d) FFTF
Q.6 Promazine can be synthesized from phenothiazine through alkylation by?
a) 3-dimethylaminobutylchloride
b) 2-dimethylaminopropylchloride
c) 3-dimethylaminopropylchloride
d) 2-dimethylaminobutylchloride
Q.7 The drug promazine hydrochloride is mainly used for?
a) Treatment of Panic attacks
b) Treatment of Schizophrenia
c) Prevention of Arrythmias
d) All of the above
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-a
2-a
3-d
4-c
5-b
6-c
7-b