RISPERIDONE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
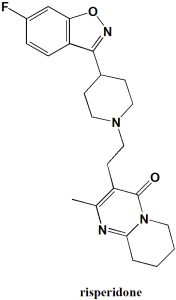
Risperidone
IUPAC nomenclature
3-[2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl]-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one.
Classification
Risperidone is an atypical antipsychotic drug.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 410.5 g/mol |
| 2 | Physical appearance | White to slightly beige powder |
| 3 | Melting point | 170°C |
| 4 | Solubility | Practically insoluble in water; soluble in methanol |
| 5 | Octanol/water partition coefficient | log Kow = 3.49 |
| 6 | Presence of ring | Benzo[d]isoxazole, piperidine, pyridopyrimidine |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
- Risperidone inhibits D2 dopaminergic receptors which leads to decrease in dopaminergic neurotransmission, which reduces the symptoms of schizophrenia.
- The drug also acts as antagonist for α1, α2 and H1 histamine receptors which helps in improving the symptoms of schizophrenia.
Structure Activity Relationship
Structure activity relationship of butyrophenones like compounds can be described as follows:
- Electron donating group at the meta-position of the phenyl ring has highest potency.
- Potency decreases on decreasing the propyl chain of butyrophenone.
- Replacing the keto oxygen with sulfur, carbon or hydroxyl group decreases the potency.
Method of synthesis
i. 1,3-difluorobenzene in acylated using 1-acetyl-4-piperidine-carbonyl chloride in dichloromethane using aluminum chloride as Lewis acid to produce 1-(4-(2,4-difluorobenzoyl)piperidin-1-yl)ethan-1-one.
ii. Then the protecting acetyl group is removed by hydrolysis in 6N hydrochloric acid on reflux to get (2,4-difluorophenyl)(piperidin-4-yl)methanone.
iii. The product is converted into oxime by reaction with hydroxylamine hydrochloride in ethanol in the presence of N,N-diethylenethanamine.
iv. Oxime undergoes cyclization to form 6-fluoro-3-(piperidin-4-yl)benzo[d]isoxazoleny refluxing with 50% potassium hydroxide solution in water.
v. Risperidone is finally obtained by alkylation of the last formed compound with 3-(2-chloroethyl)-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one on heating in dimethylformamide I the presence of sodium carbonate and potassium iodide. [1]
Therapeutic Uses
Risperidone is used for:
- Treatment of schizophrenia
- Treatment of bipolar disorder
- Treatment of irritability associated with autistic disorder
Side Effects
Side effects of Risperidone are:
- Dizziness
- Drowsiness
- Lightheadedness
- Weight gain
- Tiredness
- Drooling
- Nausea
- Muscle spasms
- Tremors
- Anxiety
- Restlessness
- Rise in blood sugar level
MCQs
Q.1 The correct statement related with the SAR of drug risperidone is?
a) Replacing the keto group with sulfur increases the activity
b) Replacing the keto group with carbon increases the activity
c) Replacing the keto group with sulfur decreases the activity
d) Replacing the keto group with sulfur or carbon decreases the activity
Q.2 Correct sequence for the True/False for correct IUPAC names of the drug can be?
- Risperidone: (Z)-3-(2-chlorothioxanthen-9-ylidene)-N,N-dimethyl-propan-1-amine
- Thioridazine: 10-[2-(1-methylpiperidin-2-yl)ethyl]-2-methylsulfanylphenothiazine
- Risperidone: 5,5-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione
- Risperidone: 5-ethyl-5-pentan-2-yl-2-sulfanylidene-1,3-diazinane-4,6-dione
a) FFTF
b) TTFF
c) FTFF
d) FFFT
Q.3 Correct statement related with the solubility of Risperidone is?
a) Practically insoluble in water
b) Slightly soluble in water
c) Soluble in water
d) Freely soluble in water
Q.4 Pick the correct statement?
a) Risperidone inhibits D2 dopaminergic receptors
b) Risperidone acts as antagonist for α1 receptors
c) Risperidone acts as antagonist for H1 histamine receptors
d) All of the above
Q.5 Which amongst the following is a therapeutic use of drug Risperidone?
a) Treatment of Schizophrenia
b) Treatment of Bipolar disorder
c) Both a) and b)
d) None of the above
Q.6 Which of the following drug and their classification are correct?
I. Risperidone: Antipsychotic drug
II. Atazanavir: HIV protease inhibitor
III. Clozapine: Nitrogen mustard
IV. Thiopental: Barbiturate sedative hypnotic
a) I, II, IV
b) I, IV
c) III, IV
d) I, II, IV
Q.7 Number of chiral carbons present in the structure of Risperidone?
a) 1
b) 2
c) 3
d) 0
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-d
2-c
3-a
4-d
5-c
6-b
7-d