DROPERIDOL Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Droperidol
IUPAC nomenclature
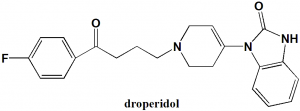
3-[1-[4-(4-fluorophenyl)-4-oxobutyl]-3,6-dihydro-2H-pyridin-4-yl]-1H-benzimidazol-2-one.
Classification
Droperidol is a buterophenone antipsychotic drug.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 379.4 g/mol |
| 2 | Physical appearance | White to light tan in color; micro-crystalline or amorphous powder |
| 3 | Melting point | 145.75°C |
| 4 | Solubility | 4.21 mg/L in water |
| 5 | Octanol/water partition coefficient | 3.5 |
| 6 | Presence of ring | Imidazole, pyridine, phenyl |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
- Droperidol is a potent Dopamine D2 receptor antagonist and minor α1-adrenoceptor antagonist.
- It causes CNS depression at subcortical levels of the brain, midbrain and brainstem reticular formation
- It also antagonizes the action of glutamic acid within the extrapyramidal system.
- It inhibits the reuptake of neurotransmitters at catecholamine receptors.
Structure Activity Relationship
Structure activity relationship of butyrophenones like compounds can be described as follows:
- Electron donating group at the meta-position of the phenyl ring has highest potency.
- Potency decreases on decreasing the propyl chain of butyrophenone.
- Replacing the keto oxygen with sulfur, carbon or hydroxyl group decreases the potency.
Method of synthesis
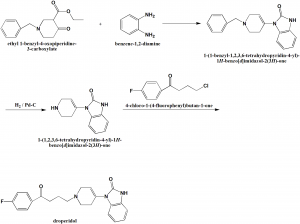
i. 1-bnzyl-3-carbethoxypiperidin-4-one is reacted with o-phenylendiamine to form 1,5-benzdiazepine.
ii. 1,5-benzdiazepine rearranges into 1-(1-benzyl-1,2,3,6-tetrahydro-4-piridyl)-2-benzymidazolone.
iii. Debenzylation of the product with hydrogen over a palladium catalyst into 1-(1,2,3,6-tetrahydro-4-piridyl)-2-benzimi-dazolon.
iv. On subsequent alkylation with 4’-chloro-4-fluorobutyrophenone gives droperidol. [1]
Therapeutic Uses
Droperidol is used for:
- Treatment of vertigo
- Decreasing hallucinations
- As an antiemetic drug
- For treatment of nausea and vomiting
Side Effects
Side effects of droperidol are:
- Hypotension
- Sedation
- Dysphoria
- Prolongation of QT interval
- Extrapyramidal side effects
MCQs
Q.1 What can be the correct IUPAC nomenclature of Droperidol?
a) 3-[1-[4-(4-fluorophenyl)-4-oxobutyl]-3,6-dihydro-2H-pyridin-4-yl]-1H-benzimidazol-2-one
b) N,N-dimethyl-9-[3-(4-methylpiperazin-1-yl)propylidene]-9H-thioxanthene-2-sulfonic acid
c) 3-(2-chlorophenothiazin-10-yl)-N,N-dimethylpropan-1-amine
d) N,N-dimethyl-3-phenothiazin-10-ylpropan-1-amine
Q.2 Which amongst the following statements is/are INCORRECT related to the SAR of Droperidol?
I. Electron donating group at the meta-position of the phenyl ring has highest potency.
II. Potency decreases on decreasing the propyl chain of butyrophenone.
III. Replacing the keto oxygen with sulfur, carbon or hydroxyl group increases the potency.
a) I, III
b) I, II
c) III
d) None
Q.3 Number of chiral carbons present in the structure of droperidol?
a) 0
b) 1
c) 2
d) 3
Q.4 Side effects of drug droperidol is/are?
a) Hypotension
b) Sedation
c) Prolongation of QT interval
d) All of the above
Q.5 Match the following drugs with their correct melting points:
| i. Droperidol | A.190-206 oC |
| ii. Oseltamivir | B. 74oC |
| iii. Umifenovir | C. 148oC |
| iv. Darunavir | D.133-137oC |
a) i-A, ii-C, iii-B, iv-D
b) i-C, ii-A, iii-D, iv-B
c) i-C, ii-A, iii-B, iv-D
d) i-B, ii-D, iii-A, iv-C
Q.6 An example of drug from class buterophenone antipsychotic drug is?
a) Droperidol
b) Chlorpromazine
c) Tiotixene
d) Both a) and c)
Q.7 The type of ring system found in droperidol?
a) Phenyl
b) Imidazole
c) Pyridine
d) All of the above
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-a
2-c
3-a
4-d
5-b
6-a
7-d