SOLIFENACIN Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
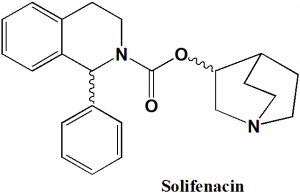
Solifenacin
IUPAC nomenclature
(3R)-1-Azabicyclo[2 2 2]oct-3-yl (1S)-1-phenyl-3,4-dihydroisoquinoline-2(1H)-carboxylate
Classification
Solifenacin is an acetylcholine antagonist. It is a muscarinic antagonist.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 362.5 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | 134-136°C |
| 4 | Solubility | Soluble in organic solvents |
| 5 | Presence of ring | Quiniclidine, quinoline, benzene |
| 6 | Number of chiral centers | 2 |
Mechanism of Action
Solifenacin functions as antagonist for muscarinic receptors M3, M1 and M2 receptors. Antagonism of M3 receptors prevents the contraction of the detrusor muscles and antagonism of the M2 receptors prevents the contraction of smooth muscle in bladder. [1]
Structure Activity Relationship
- Either R1 or R2 must be heterocyclic or carbocyclic.
- The R3 group can be hydrogen, hydroxyl, hydroxymethyl or amide.
- Most potent derivatives has X as an ester.
- X can also be either oxygen or absent completely.
- The N substituent can be quaternary ammonium salt or tertiary amine or both with different alkyl groups.
- Maximum potency obtained when the distance between the ring substituted carbons is 2 carbon units.
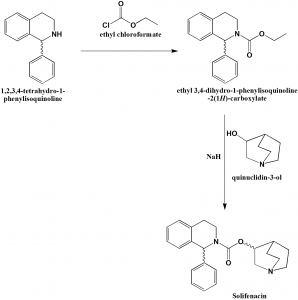
Method of synthesis
i. 1,2,3,4-tetrahydro-1-phenylisoquinoline reacts with ethyl chloroformate to give ethyl 3,4-dihydro-1-phenylisoquinoline-2(1H)-carboxylate.
ii. The latter compound on reacting with quiniclidin-3-ol, in presence of sodium hydride produces Solifenacin. [2]
Therapeutic Uses
Solifenacin is used for:
- Treatment of overactive bladder
- Reducing the frequent trips to bathroom
- Reducing the urge for frequent voids
- Reducing the leakage of urine
Side Effects
Side effects of Solifenacin are:
- Constipation
- Headache
- Dry mouth
- Blurred vision
- Dry eyes
- Tiredness
- Weakness
MCQ
Q.1 Subtypes of Muscarinic receptors antagonize by soilifenacin are?
a) M1, M2, M3
b) M2, M4, M5
c) M1, M2
d) M4, M5
Q.2 Therapeutic use of drug Solifenacin is/are?
a) Treatment of Alzheimer’s disease
b) Treatment of overactive bladder
c) Treatment of cancer
d) All of the above
Q.3 Which amongst the following are the correct statements with respect to the SAR of drug Solifenacin?
I. Either R1 or R2 must be heterocyclic or carbocyclic.
II. The R3 group can be hydrogen, hydroxyl, hydroxymethyl or amide.
III. Most potent derivatives has X as an ester.
IV. ‘X’ can also be either oxygen or absent completely.
a) I, IV
b) I, II, IV
c) I, II, III, IV
d) II, III, IV
Q.4 Type of ring structures present in the structure of solifenacin?
a) Quiniclidine
b) Quinoline
c) Benzene
d) All of the above
Q.5 Correct sequence for the True/False for the physiochemical properties of the drug solifenacin?
I. Molecular weight is 363.5 gm/mol
II. Present in solid form
III. Melting point is between 134-136°C
IV. Soluble in organic solvents
a) TFTF
b) TTTT
c) FFFF
d)FFFT
Q.6 Correct statements for the IUPAC nomenclatures of the are?
I. Solifenacin: (3R)-1-Azabicyclo[2 2 2]oct-3-yl (1S)-1-phenyl-3,4-dihydroisoquinoline-2(1H)-carboxylate
II. Zaleplon: N-(3-(3-cyanopyrazolo[1,5-a] pyrimidin-7-yl)phenyl)-N-ethylacetamide
III. Alprazolam: 7-Chloro-1,3-dihydro-1-methyl-5-phenyl-3H-1,4-benzodiazepin-2-one
IV. Diazepam: 8-Chloro-1-methyl-6-phenyl-4H-[1,2,4]triazolo[4,3-a] [1,4]benzodiazepine
a) II, IV
b) I, II
c) I, III, IV
d) I, II, III, IV
Q.7 Match the following drugs with their correct classifications-
| i. Solifenacin | A. Barbiturate sedative-hypnotic |
| ii. Thiobarbital | B. Benzodiazepine sedative-hypnotic |
| iii. Diazepam | C. Acetylcholine antagonist |
| iv. Zaleplon | D. Nonbenzodiazepine sedative-hypnotic |
a) i-C, ii-A, iii-B, iv-D
b) i-A, ii-B, iii-C, iv-D
c) i-D, ii-B, iii-A, iv-C
d) i-D, ii-B, iii-C, iv-A
Participate in Free Online Test for GPAT, Pharmacist,Drug Inspector
ANSWERS
1-a
2-b
3-c
4-d
5-b
6-b
7-a
REFERENCES
[1] Morales-Olivas FJ, Estañ L. Solifenacin pharmacology. Arch Esp Urol. 2010;63(1):43-52. [2] Serrano JP, Camps P, inventors; Medichem SA, assignee. Process for the synthesis of solifenacin. United States patent US 7,741,489. 2010 Jun 22.