Solubility: Miscibility and MCQs for NEET GPAT, NIPER, Pharmacist and Drug Inspector exam
Solubility of Liquid in Liquid: Frequently two or more liquids are mixed together in the preparation of pharmaceutical products (e.g. aromatic waters, spirits, elixirs, lotions, sprays, and medicated oils). Liquid–liquid systems can be divided into two categories according to the solubility of the substances in one another:
- Complete miscibility 2. Partial miscibility.
Complete Miscibility- Polar and semipolar solvents, such as water and alcohol, alcohol and acetone, are said to be completely miscible because they mix in all proportions. Nonpolar solvents such as benzene and CCl4 are also completely miscible. These liquids are miscible because the broken attractive forces in both pure liquids are re-established in the mixture.
Partial Miscibility: When water and phenol are mixed, two liquid layers are formed each containing some of the other liquid in the dissolved state. It is possible to calculate the composition of each component in the two conjugate phases and the relative amount of each phase from the tie lines that cut the binodal curve.
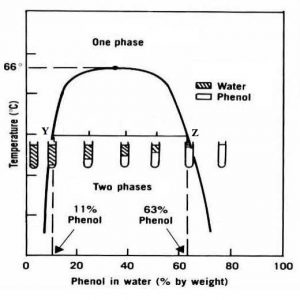
Fig 1- Phenol water system phase diagram(taken from phenol-water system carnotcycle)
Partially miscible liquids are influenced by temperature. The two conjugate phases changed to a homogenous single phase at the critical solution temperature (or upper consolute temperature). Some liquid pairs (e.g. trimethylamine and water) exhibit a lower consolute temperature, below which the two members are soluble in all proportions and above which two separate layers form.
Few mixtures (e.g. nicotine and water) show both an upper and a lower consolute temp. with an intermediate temp. region in which the two liquids are only partially miscible. A final type exhibits no critical solution temperature (e.g. ethyl ether and water shows partial miscibility over the entire temperature range at which the mixture exists.
Solubility of Gas in Liquid:
The Mass of Gas Molecules
- The solubility of gas molecules typically increases with increasing mass of the gas molecules.
- The larger the mass of gas molecules, the stronger London and Debye forces is between gas and solvent molecules.
Henry’s law
- The effect of the pressure on the solubility of a gas is expressed by Henry’s law
- Henry’s law states, “In a very dilute solution at constant temperature, the concentration of dissolved gas is proportional to the partial pressure of the gas above the solution at equilibrium” • The partial pressure of the gas is obtained by subtracting the vapor pressure of the solvent from the total pressure above the solution.
- If C2 is the concentration of the dissolved gas in grams per liter of solvent and p is the partial pressure in millimeters of the undissolved gas above the solution,
- Henry’s relationship may be written as C2 = σp
- σ=1/k solubility coefficient.
Temperature
- Gases decrease in solubility with an increase in temperature.
- Increasing temperature causes an increase in kinetic energy of gas molecules which leads to breakdown of intermolecular bonds and gas escaping from solution.
- E.g. Carbon dioxide gases escape faster from a carbonated drink as the temperature increases.
Presence of Salts
- Dissolved gases are often liberated from solutions by the introduction of an electrolyte (e.g. NaCl) and sometimes by a non electrolyte (e.g. sucrose)
- This phenomenon is known as SALTING OUT.
pH
- Systems of solids in liquids include the most frequent and important type of pharmaceutical solutions.
- Most drugs belong to the class of weak acids and bases. They react with strong acids or bases to form water soluble salts.
- Acidic drugs (e.g. NSAIDs), are more soluble in solutions where the ionized form is the predominant.
- Basic drugs(e.g. ranitidine), are more soluble in acidic solutions where the ionized form of the drug is predominant.
Multiple choice questions(MCQs)
1.The solubility of the substance depends on
a)Solvent
b)Temperature
c)Pressure
d)All of the above
2.At a specified temperature, maximal amount of solute that can dissolve in an amount of solvent is known as
a)Solubility
b)Dissolution
c)Diffusion
d)Capacity
3.Solubility curve is a curve drawn between
a)Solubility and temperature
b)Solubility and pressure
c)Solubility and mole fraction
d)None of the above
4.The solubility of gas ____ with rising temperature
a)Increase
b)Decrease
c)Remain constant
d)None of the above
5.Liquid–liquid systems can be divided into which 0f the following categories according to the solubility of the substances in one another?
a)Complete miscibility
b)Partial miscibility
c)Immiscibility
d)a) and b)
6.Which of the following show complete miscibility?
a)Polar and semipolar solvents
b)Water and alcohol
c)Alcohol and acetone
d)All of the above
7.Which nonpolar solvent is completely miscible?
a)Polar and semipolar solvents
b)Water and alcohol
c)Alcohol and acetone
d)Benzene and CCl4
8.Partially miscible liquids are influenced by
a)temperature
b)pressure
c)pH
d)time
9.Ethyl alcohol is a
a)Semi-polar solvent
b)Polar solvent
c)Non-polar solvent
d)None of the above
10.In these all are polar solvent except one
a)Glycols
b)Methyl alcohols
c)Acetone
d)Water
11.When solute or ions dissolved in a solvent, then solute get surrounded by solvent molecules, this process is known as
a)Solvation
b)Dissolution
c)Solubility
d)All
12.CST represents for
a)Consolute temperature
b)Critical solubility temperature
c)Critical solution temperature
d)None
13.In a solution the solute
a)Refers to the temperature
b)Is what is dissolved
c)Refers to the polarity
d)Does the dissolving
14.The effect of the pressure on the solubility of a gas is expressed by
a)Raoults law
b)Daltons law
c)Henrys law
d)Boyles law
15.Henry’s relationship may be written as
a)C2 = σp
b)C = σp
c)V = σp
d)E = σp
Solutions:
- d)All of the above
- a)Solubility
- a)Solubility and temperature
- a)Increase
- d)a) and b)
- d)All of the above
- d)Benzene and CCl4
- a)temperature
- b)Polar solvent
- c)Acetone
- a)Solvation
- c)Critical solution temperature
- b)Is what is dissolved
- c)Henrys law
- a)C2 = σp
References:
1. Martins Physical Pharmacy, 6th edition 2011, page no. 332-355.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test