Solubility: Three component systems and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam
A phase is the part whose physical and chemical properties are completely equal and homogenous. it is separated from other parts of the system by interfaces. A system containing water and its vapor is a two-phase system. An equilibrium mixture of ice, liquid water, and water vapor is a three- phase system.
A phase may be gas, liquid or solid. A gas or a gaseous mixture is a single phase. Completely miscible liquids constitute a single phase. In an immiscible liquid system, each layer is counted as a separate phase. Every solid constitutes a single phase except when a solid solution is formed. A solid solution is considered as a single phase. Each polymorphic form constitutes a separate phase.
Phase diagram (also known as Equilibrium Diagrams) shows the multisystem state changes with the temperature, pressure, composition and other intensive properties. The simplest phase diagrams are pressure-temperature diagrams of a single simple substance.
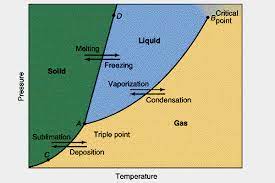
Fig 1 – Phase diagram(taken from Phase rule and Phase equilibria-ppt video)
The Gibbs phase rule: To understand and define the state of a phase, knowledge of several independent variables is required. Independent variables (also called Intensive variables) are the variables that do not depend on the volume or size of the phase, e.g. temperature, pressure, density, boiling point and concentration. The Gibbs phase rule is expressed as follows: F= C – P+ 2 F is the number of degrees of freedom of the system which is the least number of intensive variables required to define the system completely. C: number of components, P: number of phases present.
Three component system containing liquid phases: For three component systems:
C = 3, P = 1 at least F =C – P + 2 F = 3 – P + 2 = 5 – P so F is 4 at most Four variables are required: temperature, pressure, and two composition.
If the temperature and pressure is both fixed: C = 3 F = C – P ⟹ F = 3 – P P = 1 at least, so F is 2 at most Only two composition is required.
Multiple choice questions(MCQs)
1.The temperature at which two conjugate solutions merge into one another to form one layer is called
a)Upper consolute temperature
b)Critical saturation temperature
c)Distillation temperature
d)Partition temperature
2.The part whose physical and chemical properties are completely equal and homogenous is called
a)phase
b)interface
c)surface
d)all of the above
3.Which of the following is a two-phase system?
a)water and its vapor
b)An equilibrium mixture of ice, liquid water, and water vapor
c)both of these
d)none of these
4.For the study of distribution law the two solvents should be
a)Non miscible
b)Miscible
c)Volatile
d)Reacting with each other
5.An equilibrium mixture of ice, liquid water, and water vapor is a
a)one component system
b)two component system
c)three component system
d)all of the above
6.A phase may be
a)gas
b)liquid
c)solid
d)all of the above
7.A gas or a gaseous mixture is a
a)single phase
b)two phase
c)three phase
d)all of the above
8.The solubility of solids generally rises with
a)Increase in temperature
b)Decrease in temperature
c)Increase in volume of the solvent
d)None of the above
9.When a saturated solution prepared at a high temperature is cooled, we get
a)Super cooled solution
b)Super saturated solution
c)An equilibrium mixture
d)One molar solution
10.Completely miscible liquids constitute a
a)single phase
b)two phase
c)three phase
d)all of the above
11.Phase diagram is also known as
a)interface diagram
b)equilibrium diagrams
c)rheograms
d)none of the above
12.Phase diagram (also known as Equilibrium Diagrams) shows the multisystem state changes with the
a)temperature
b)pressure
c)composition
d)all of the above
13.The Gibbs phase rule is expressed as follows:
a)F= C – P+ 2
b)F= C + P+ 2
c)F= C – P- 2
d)F= C + P- 2
14.For three component systems C is equal to
a)1
b)2
c)3
d)0
15.For three component systems P is equal to
a)1
b)2
c)3
d)0
Solutions:
- a)Upper consolute temperature
- a)phase
- c)both of these
- a)Non miscible
- c)three component system
- d)all of the above
- a)single phase
- a)Increase in temperature
- b)Super saturated solution
- a)single phase
- b)equilibrium diagrams
- d)All of the above
- a)F= C – P+ 2
- c)3
- a)1
References:
- Martins Physical Pharmacy, 6th edition 2011, page no. 332-355.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test