Suppositories: Manufacturing procedure and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam
Formulation variables: Formulation variables that are generally considered include (a) the nature and form of the active principle (esters, salts, complexes, etc.), (b) the physical state, particle dimensions, and the specific surface of the product, (c) the solubility of the drug in various bases, (d) the presence or absence of adjuvants added to the active principle, (e) the nature and type of dosage form in which the active principle is incorporated, and (f) pharmaceutical procedures used in the preparation of the dosage form.
Physical State – An active drug can be a solid, liquid, or semisolid in nature. For solids, the drug’s particle size may be very important, especially if the drug is not very water soluble; the increase in surface area resulting from decreased particle size can serve to enhance its activity. For liquids, it is necessary to take up the liquid into the suppository base using one of several techniques such as forming an emulsion, adding a drying powder, or adding a suitable thickening agent when the liquid is mixed with the suppository base. For the semisolids or paste-type drugs, it can be either mixed with a solid that will serve to thicken the drug prior to mixing with the base or mixed with the base to which a thickener is added.
Particle Size – If a drug is readily soluble, the influence of particle size may be minimal. For highly water-soluble drugs, the tendency will be to dissolve and migrate to the rectal barrier. For poorly water-soluble drugs, the dissolution rate will be slower, and a reduction in particle size may increase the rate of dissolution by exposing a greater surface area. This can also be affected by the nature of the suppository base.
Solubility – Increased solubility of the active in the base can improve product homogeneity; however, it may also delay the release of the active if there is too great an affinity of the drug for the suppository vehicle. If the active ingredient is insoluble in the base, as is the case when a “suspension” or “emulsion” is formed, this poses different problems. It is necessary to maintain homogeneity of the total mixture; this can usually be obtained by constant agitation of the mixture during processing and filling. Often times, it is best to select a temperature just above the melting point of the suppository mixture where the mixture is thick but still pourable.
Viscosity – Viscosity considerations are also important in the preparation of the suppositories and the release of the drug. If the viscosity of a base is low, it may be necessary to add a suspending agent such as silica gel to ensure that the drug is uniformly dispersed
until solidification occurs. When preparing the suppository, the pharmacist should stir the melt constantly and keep it at the lowest possible temperature to maintain a high viscosity. After the suppository has been administered, the release rate of the drug may be slowed if the viscosity of the base is very high. This is because the viscosity causes the drug to diffuse more slowly through the base to reach the mucosal membrane for absorption.
Brittleness – Brittle suppositories can be difficult to handle, wrap, and use. Cocoa butter suppositories are usually not brittle unless the percentage of solids present is high. In general, brittleness results when the percentage of nonbase materials exceeds about 30%. Synthetic fat bases with high stearate concentrations or those that are highly hydrogenated are typically more brittle. Shock cooling also causes fat and cocoa butter suppositories to crack. This condition can be prevented by ensuring that the temperature of the mold is as close to the temperature of the melted base as possible. Suppositories should not be placed in a freezer, which also causes shock cooling.
Volume contraction – Bases, excipients, and active ingredients generally occupy less space at lower temperatures than at higher temperatures. When preparing a suppository, the pharmacist pours hot melt into a mold and allows the melt to cool. During this cooling process, the melt has a tendency to contract in size. This makes it easier to release the suppository from the mold, but it may also produce a cavity at the back, or open end, of the mold. Such a cavity is undesirable and can be prevented if the melt is permitted to approach its congealing temperature immediately before it is poured into the mold. It is advisable to pour a small amount of excess melt at the open end of the mold to allow for the slight contraction during cooling. Scraping with a blade or spatula dipped in warm water will remove the excess after solidification, but care must be taken not to remove the metal from the mold. The heated instrument can also be used to smooth out the back of the suppository.
Drug release rates – General approximate drug release rates as they relate to the drug and base characteristics are summarized as follows:
Table 1 – General approximate drug release rates
| DRUG:BASE CHARACTERISTICS |
APPROXIMATE DRUG RELEASE RATE |
| Oil-soluble drug:Oily base | Slow release; poor escaping tendency |
| Water-soluble drug:Oily base | Rapid release |
| Oil-soluble drug:Water-miscible base | Moderate release |
| Water-miscible drug:Water-miscible base | Moderate release;based on diffusion;all water soluble |
Manufacturing of suppositories:
- Hand rolling
- Fusion method
- Cold compression
Hand rolling – It is the oldest and simplest method of suppository preparation and may be used when only a few suppositories are to be prepared in a cocoa butter base. It has the advantage of avoiding the necessity of heating the cocoa butter. A plastic-like mass is prepared by triturating grated cocoa butter and active ingredients in a mortar. The mass is formed into a ball in the palm of the hands, then rolled into a uniform cylinder with a large spatula or small flat board on a pill tile. The cylinder is then cut into the appropriate number of pieces which are rolled on one end to produce a conical shape. Effective hand rolling requires considerable practice and skill.
Fusion method – It involves following steps:
- Melting the suppository base
- Dispersing or dissolving the drug in the melted base.
- The mixture is removed from the heat and poured into a suppository mold.
- Allowing the melt to congeal.
- Removing the formed suppositories from the mold.
The fusion method can be used with all types of suppositories and must be used with most of them. Small scale molds are capable of producing 6 or 12 suppositories in a single operation. Industrial molds produce hundreds of suppositories from a single molding.
Cold compression – Compression molding is a method of preparing suppositories from a mixed mass of grated suppository base and medicaments which is forced into a special compression mold using suppository making machines.
- The suppository base and the other ingredients are combined by thorough mixing.
- The friction of the process causing the base to soften into a past-like consistency. The friction of the process causing the base to soften into a past-like consistency.
- On large scale, mechanically operated kneading mixers and a warmed mixing vessel may be applied.
- In the compression machine, the suppository mass is placed into a cylinder which is then closed.
- Pressure is applied from one end to release the mass from the other end into the suppository mold or die.
- When the die is filled with the mass, a movable end plate at the back of the die is removed and when additional pressure is applied to the mass in the cylinder, the formed suppositories are ejected.
- The end plate is returned, and the process is repeated until all of the suppository mass has been used.
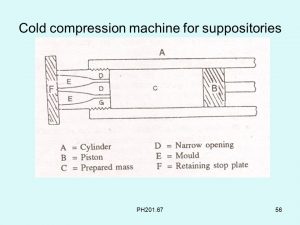
Figure 1 – Cold compression molding machine
Multiple choice questions:
1.Formulation variables that are generally considered in suppositories include
a)the nature and form of the active principle
b)the physical state, particle dimensions, and the specific surface of the product
c)the solubility of the drug in various bases
d)all of these
2.For liquids, it is necessary to take up the liquid into the suppository base using one of several techniques such as
a)forming an emulsion
b)adding a drying powder
c)adding a suitable thickening agent
d)all of these
3.For highly water-soluble drugs, the tendency will be to dissolve and migrate to the rectal barrier.
a)true
b)false
4.For poorly water-soluble drugs, the dissolution rate will be slower, and a reduction in particle size may increase the rate of dissolution by exposing a greater surface area.
a)true
b)false
5.If the viscosity of a base is low, it may be necessary to add
a)suspending agent
b)emulsifying agent
c)wetting agent
d)all of these
6.Which of the following bases are more brittle?
a)Synthetic fat bases with high stearate concentrations
b)those that are highly hydrogenated
c)both of these
d)none of these
7.Approximate drug release rate of Oil-soluble drug:Oily base is
a)Slow release
b)Rapid release
c)Moderate release
d)Based on diffusion
8.Approximate drug release rate of Water-soluble drug:Oily base is
a)Slow release
b)Rapid release
c)Moderate release
d)Based on diffusion
9.Approximate drug release rate of Oil-soluble drug:Water-miscible base is
a)Slow release
b)Rapid release
c)Moderate release
d)Based on diffusion
10.Which of the following methods are used for manufacturing of suppositories?
a)Hand rolling
b)Fusion method
c)Cold compression
d)All of these
11.Which of the following is oldest method for manufacturing of suppositories?
a)Hand rolling
b)Fusion method
c)Cold compression
d)All of these
12.Which of the following is second step in fusion method?
a)Melting the suppository base
b)Allowing the melt to congeal
c)Removing the formed suppositories from the mold
d)Dispersing or dissolving the drug in the melted base
13.Small scale molds are capable of producing how many suppositories in a single operation?
a)1 or 5
b)6 or 12
c) 15 or 20
d)more than 20
14.In the compression machine, the suppository mass is placed into a cylinder which is then kept open.
a)true
b)false
15.Compression molding is a method of preparing suppositories from a mixed mass of grated suppository base and medicaments which is forced into a special compression mold using suppository making machines.
a)true
b)false
Solutions:
- d)all of these
- d)all of these
- a)true
- a)true
- a)suspending agent
- c)both of these
- a)Slow release
- b)Rapid release
- c)Moderate release
- d)All of these
- a)Hand rolling
- d)Dispersing or dissolving the drug in the melted base
- b)6 or 12
- b)false
- a)true
References:
- Ansel’s Pharmaceutical Dosage Forms and Drug Delivery systems, 10th edition, page no. 377-383.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test