TOLMETIN Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
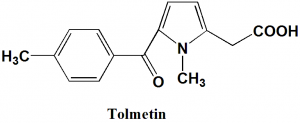
Tolmetin
IUPAC nomenclature
[1-methyl-5-(4-methylbenzoyl)-1H-pyrrol-2-yl]acetic acid.Classification
- Nonsteroidal anti-inflammatory drug
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 257.28 g/mol |
| 2 | Physical appearance | Crystals from acetonitrile |
| 3 | Melting point | 156°C |
| 4 | Solubility | 222 mg/L |
| 5 | Octanol/water partition coefficient | 2.79 |
| 6 | Presence of ring | Pyrrole, phenyl |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
- Prostaglandin synthetase enzyme is inhibited by tolmetin which lowers the plasma level of prostaglandin in man. This is responsible for the anti-inflammatory action of the drug.
Structure Activity Relationship
SAR of Tolmetin can be summarized as follows:
- Replacement of the 5-p-toluoyl group with p-chlorobenzoyl moiety have little changes on the activity of drug.
- 4-methyl-5-p-chlorobenzoyl analog is 4 times more potent then tolmetin.
- Substituting the chloro group with the p-methyl group blocks the oxidative metabolism which increases the duration of action by 1 day.
- Propionic acid analogue is less potent.
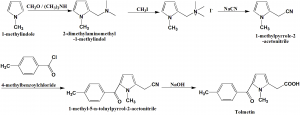
Method of synthesis
i. 1-methylindole is aminomethylated using formaldehyde and dimethylamine to form 2-dimethylaminomethyl-1-methylindol.
ii. Last is methylated by methyl iodide to produce the corresponding quarternary salt.
iii.Eaction of the above formed compound with sodium cyanide produces 1-methylpyrrole-2-acetonitrile.
iv. The product is then acylated at the free α-position of te pyrole ring by 4-methylbenzoylchloride in the presence o aluminum chloride to give 1-methyl-5-n-toluylpyrrol-2-acetonitrile.
v. The last further undergoes alkaline hydrolysis to finally produce tolmetin. [2]
Therapeutic Uses
Tolmetin is used for:
- Reducing pain, joint stiffness and swelling due to arthritis.
- Treatment of juvenile rheumatoid arthritis
Side Effects
Side effects of Tolmetin are:
- Nausea
- Vomiting
- Heartburn
- Diarrhea
- Upset stomach
- Dizziness
- Headache
- Hypertension
- Ringing in ears
- Mood changes
- Symptoms of heart failures
- Kidney problems
- Stiff neck
- Liver disease
- Allergic reactions
MCQs
Q.1 Choose the correct statements related with the physicochemical properties of drug Tolmetin.
I. Molecular weight = 147.0 gm/mol
II. It forms crystals from Acetonitrile
III. Melting point is 156 oC
a) I, III
b) II, III
c) III
d) I, II
Q.2 Match the following of the drugs with their correct Brand names.
| i. Tolmetin | A. Navane
|
| ii. Thiotixene | B. Halidol
|
| iii. Haloperidol | C. Tolectin
|
| iv. Chloraquine | D. Aralen |
a) i-C, ii-A, iii-B, iv-D
b) i-D, ii-A, iii-C, iv-B
c) i-D, ii-A, iii-B, iv-C
d) i-A, ii-C, iii-B, iv-D
Q.3 Tolmetin inhibits
I. DNA polymerase enzyme
II. DNA dependent RNA polymerase enzyme
III. Prostaglandin synthetase enzyme
IV. Nucleosidases
a) I , II
b) III
c) III , IV
d) IV
Q.4 Correct sequence for True/false for the classification of the drug can be?
- Tolmetin: Opioid Antagonist
- Asenapine: Benzodiazepine
- Clozapine: Benzazepine
- Haloperidol: buterophenone
a) TFFT
b) TTTT
c) TTFF
d) FFTT
Q.5 Find the INCORRECT statement amongst the following related with the SAR of Tolmetin?
I. Replacement of the 5-p-toluoyl group with p-chlorobenzoyl moiety have little changes on the activity of drug.
II. 4-methyl-5-p-chlorobenzoyl analog is 4 times more potent then tolmetin.
III. Substituting the chloro group with the p-methyl group blocks the oxidative metabolism which increases the duration of action by 1 day.
IV. Propionic acid analogue is more potent
Q.6 Number of chiral carbons present in the structure of tolmetin?
a) 0
b) 1
c) 2
d) 3
Q.7 Side effect of drug Tolmetin include?
a) Symptoms of heart failure
b) Upset stomach
c) Kidney problems
d) All of the above
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-b
2-a
3-b
4-d
5-d
6-a
7-d