TRIFLUOPERAZINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
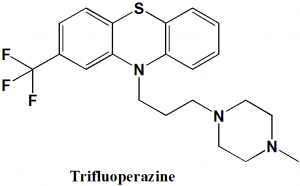
Trifluoperazine
IUPAC nomenclature
10-[3-(4-methylpiperazin-1-yl)propyl]- 2-(trifluoromethyl)-10H-phenothiazine.
Classification
Trifluoperazine is phenothiazine antipsychotic drug.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 407.5 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | 232°C |
| 4 | Solubility | Freely soluble in water |
| 5 | Octanol/water partition coefficient | 5.03 |
| 6 | Presence of ring | Phenothiazine, piperazine |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
- Trifluoperazine blocks postsynaptic dopaminergic D1 and D2 receptors in brain.
- Trifluoperazine also blocks α-adrenergic effects
- Depresses the release of hypothalamic and hypophyseal hormones
- Depresses the reticular activating system
- Due to these, basal metabolism, temperature, wakefulness, vasomotor tone and emesis of the body is affected.
Structure Activity Relationship
Structure activity relationship of phenothiazine can be described as follows:
- Tilting of side chain towards ring A grants favorable Vander Waal’s interaction of the side chain. This interaction decides the potency of the drug towards the dopamine receptors.
- Optimal neuroleptic activity occurs when the ring A substituent is in the 2nd-position.
- A trifluoromethyl substituent provides a greater number of favorable Van der Waal’s contacts with the side chain than the chlorine substituent. Thus, phenothiazne with trifluoromethyl substituents are more potent than those with chlorine substituent.
- A piperazine side chain provides more Van der Waal’s contacts with 2-substituent than the alkylamino side chain. Thus, piperizine phenothiazine are more potent in antischizophrenic effects than alkylamino phenothiazines.
- Hydroxyethylpiperazine side chain phenothiazines displays more favorable Van der Waal’s interactions with ring A than simple piperazines.
- In the thioxanthene and xanthenes containing ring systems, the cis forms are more potent neuroleptics than the trans isomers.
- Phenothiazine analogues having the presence of exolytic double bond are more potent than the corresponding compounds lacking the exolytic double bonds. [1]
Method of synthesis
Trifluoperazine can be synthesized by reacting 2-(trifluoromethyl)-10H-phenothiazine with 1-(3-chloropropyl)-4-methylpiperazine in the presence of sodium amide.
Therapeutic Uses
Trifluoperazine is used for:
- Treatment of schizophrenia
- Treatment of psychotic disorders
- Reducing aggressiveness
- Decreasing hallucinations
- Short-term treatment of anxiety
Side Effects
Side effects of trifluoperazine are:
- Dizziness
- Drowsiness
- Lightheadedness
- Dry mouth
- Weight gain
- Blurred vision
- Constipation
- Mood changes
- Tremors
- Severe muscle spasm
MCQs
Q.1 “10-[3-(4-methylpiperazin-1-yl)propyl]- 2-(trifluoromethyl)-10H-phenothiazine” is the IUPAC nomenclature of which drug?
a) Trifluoperazine
b) Promazine
c) Fluoxetine
d) Quetiapine
Q.2 The approximate molecular weight of the drug Trifluoperazine is?
a) 152.6 gm/mol
b) 407.5 gm/mol
c) 578.6 gm/mol
d) 446 gm/mol
Q.3 Match the following with correct classifications of the drugs.
| i. 2,5-dimethoxy-4-iodoamphetamine | A. 5-HT receptor antagonist |
| ii. Granisetron | B. Hallucinogenic agent |
| iii. Almotriptan | C. 5-HT receptor agonist |
| iv. Trifluoperazine | D. Phenothiazine |
a) i-B, ii-A, iii-C, iv-D
b) i-D, ii-A, iii-B, iv-C
c) i-A, ii-B, iii-D, iv-C
d) i-D, ii-B, iii-A, iv-C
Q.4 Choose the correct statements related with the Mechanism of action of drug trifluoperazine?
I. Blocks Dopamine D1 receptors
II. Blocks Dopamine D2 receptos
III. Stimulates α-adrenoceptors
IV. Stimulates ß-adrenoceptor
a) I, II
b) III, IV
c) I, II, III
d) II, III, IV
Q.5 Correct sequence for True and False for the given statements related with the SAR of drug trifluoperazine?
- Phenothiazine analogues having the presence of exolytic double bond are more potent than the corresponding compounds lacking the exolytic double bonds.
- Optimal neuroleptic activity occurs when the ring A substituents is in the 2nd-position.
- A trifluoromethyl substituent provides a greater number of favourable Van der Waal’s contacts with the side chain than the chlorine substituent. Thus, phenothiazne with trifluoromethyl substituents are therefore less potent than those with chlorine substituents.
- A piperazine side chain provides lesser Van der Waal’s contacts with 2-substituent than the alkylamino side chain. Thus, piperizine phenothiazine are more potent in antischizophrenic effects than alkylamino phenothiazines.
a) TFTT
b) TTFF
c) FFTT
d) FFTF
Q.6 Trifluoperazine can be synthesized by the interaction of?
a) Phenothiazine derivative and alkylamide
b) Piperazine derivative and Sulfuric acid
c) Phenothiazine derivative and Piperazine derivative
d) None of the above
Q.7 The drug Trifluoperazine is mainly used for?
a) Treatment of Psychotic disorders
b) Treatment of Schizophrenia
c) Reducing aggressiveness
d) All of the above
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-a
2-b
3-a
4-a
5-b
6-c
7-d