TROSPIUM CHLORIDE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
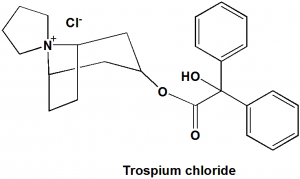
Trospium chloride
IUPAC nomenclature
3‑(2‑Hydroxy-2,2‑diphenylacetoxy)spiro[bicyclo[3.2.1]octane-8,1’‑pyrrolidin]-1’‑ium chloride.
Classification
Trospium is an antispasmodic agent.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 428 g/mol |
| 2 | Physical appearance | Colorless to slightly yellow, fine crystalline solid. |
| 3 | Melting point | 255-258°C. |
| 4 | Solubility | 1 gm/2ml in water |
| 5 | Presence of ring | Benzene and other ring structure present |
| 6 | Number of chiral centers | 3 |
Mechanism of Action
- Trospium acts as antagonist for the acetylcholine for muscarinic receptors in cholinergically innervated organs.
- In the bladder, its parasympatholytic action decreases the tonus of smooth muscles. [1]
Structure Activity Relationship
- Either R1 or R2 must be heterocyclic or carbocyclic.
- The R3 group can be hydrogen, hydroxyl, hydroxymethyl or amide.
- Most potent derivatives has X as an ester.
- X can also be either oxygen or absent completely.
- The N substituent can be quaternary ammonium salt or tertiary amine or both with different alkyl groups.
- Maximum potency obtained when the distance between the ring substituted carbons is 2 carbon units.
Method of synthesis
i. (1) undergoes chlorination to prouduce (2).
ii. (2) reacts with (3) in presence of pyridine and chloroform to give (4).
iii. (4) in presence of K2CO3 and water produces (5).
iv. (5) undergoes reduction to give (6).
v. (6) reacts with (7) to produce trospium chloride.
Therapeutic Uses
Trospium chloride is used for:
- Treatment of overreactive bladder
- Reducing leakage of urine
- Reducing the feeling of needing to urinate
- Decreases the frequent trips to bathrooms
Side Effects
Side effects of trospium chloride are:
- Dry mouth
- Dizziness
- Stomach upset
- Dry nose
- Blurred vision
- Drowsiness
MCQ
Q.1 3‑(2‑Hydroxy-2,2‑diphenylacetoxy)spiro[bicyclo[3.2.1]octane-8,1′‑pyrrolidin]-1′‑ium chloride” is the IUPAC nomenclature of which drug?
a) Acetylcholine
b) Cevimeline
c) Trospium chloride
d) Carvedilol
Q.2 Molecular weight of Trospium chloride is?
a) 146.21 gm/mol
b) 199.32 gm/mol
c) 428 gm/mol
d) 406.5 gm/mol
Q.3 Match the following with correct classifications of the drugs.
| i. Cavedilol | A. Antispasmodic agent |
| ii. Trospium chloride | B. Ach mimetic |
| iii. Cevimeline | C. Mixed α/ß blocker |
| iv. Metocurine | D. Nicotinic antagonist |
a) i-,B ii-,C iii-A, iv-D
b) i-D, ii-A, iii-C, iv-B
c) i-C, ii-A, iii-B, iv-D
d) i-B, ii-A, iii-C, iv-A
Q.4 Trospium chloride mechanism of action depends on?
a) Antagonism of acetylcholine for muscarinic receptors
b) Agonism of acetylcholine for muscarinic receptors
c) Antagonism of acetylcholine for nicotinic receptors
d) Agonism of acetylcholine for nicotinic receptors
Q.5 Correct sequence for True and False for the given statements related with the SAR of drug trospium chloride.
- Either R1 or R2 must be heterocyclic or carbocyclic.
- The R3 group can be hydrogen, hydroxyl,hydroxymethyl or amide.
- Most potet derivatives has X as an ester.
- X can also be either oxygen or absent completely.
a) TTFF
b) FTFT
c) TTTT
d) FFTF
Q.6 Number of chiral centers present in trospium chloride?
a) 1
b) 2
c) 3
d) 4
Q.7 The drug trospium chloride is mainly used for?
a) Symptoms of dry mouth
b) Alzhiemer’s disease
c) Treatment of overreactive bladder
d) None of the above
ANSWERS
1-c
2-c
3-c
4-a
5-c
6-c
7-c
REFERENCES
[1] Pak RW, Petrou SP, Staskin DR. Trospium chloride: a quaternary amine with unique pharmacologic properties. Current urology reports. 2003 Nov 1;4(6):436-40.