Ultra Violet Spectroscopy: Electronic Transition And Excitation process and Question Answers Discussion
¤ What is uv- visible spectroscopy?
It is molecular spectroscopy in which we study interaction between molecules and UV – visible radiation.
UV- Visible spectroscopy is work on absorption phenomenon.
Basic:-
Ultra Violet spectroscopy is concerned with the study of absorption of UV radiation which range from 200nm to 400nm.
Visible spectroscopy is concerned with the study of the absorption of visible radiation which range from 400nm to 800nm.
In both spectroscopy sample is absorbed radiation energy and valence electrons are goes ground state to excited state.
There are mainly three types of electron
- σ – electron :- These are the one present in saturated compound. Such electron do not absorb near UV but absorb vacuum UV radiation.
- π electron :- These electron are present in unsaturated compound double or triple bond.
- N electron :- These are non bonded electron which are not involved in any bonding lone pair of electron like in S,O,N and Halogen.
PRINCIPLE
It is mainly work on absorption phenomenon. We measure the absorption of light by molecules that are in a gas or vapour state or dissolved molecules in spectrophotometry.
Investigation the absorption of the difference substance wave length is 190 nm to 780 nm.
Electronic Transition And Excitation process :-
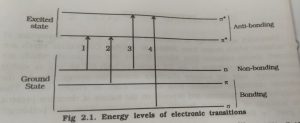
It was stated earlier that σ,π, and n electron are present in a molecule and can be excited from the ground state by the absorption of UV radiation.
There are mainly four types of transition are seen
- σ—> σ∗
- π –> π∗
- n —> π∗
- n –> σ∗
Energy level of electronic transition
Type of electronic transition
1. n⇒π∗ :- This electron transition required lowest energy.
The peak of this transition is called R band.
Wavelength is approximately 200 nm.
This type of peak can be seen in compound where n electron is present in a compound containing double bond or triple bond eg. Aldehyde or ketones
2. π⇒π∗ :- This type of transition give B,E, and k band.

Wavelength is approximately more than 200 nm.
3. n⇒σ∗
In this transition approximately 175 nm wavelength are seen.
When any hetero atom is present in the saturated compound this transition is occur.
This transition is also seen in water, chloroform, alcohol.
4.σ⇒σ∗
This type transition required the highest energy.
The peak is seen in 100 nm to 150 nm
Example of this transition is hydrocarbon.
MCQ.
1. UV SPECTROSCOPY working on which phenomenon ?
A. Absorption
B. Elution
C. A and B
D. None of this
2. Ultra Violet spectroscopy wavelength is ?
A. 200 nm to 300nm
B. 200nm to 400nm
C. 400nm to 800 nm
D. All of the above
3. Visible spectroscopy wavelength range is ?
A. 200 nm to 300nm
B. 200nm to 400nm
C. 400nm to 800 nm
D. All of the above
4. n⇒σ∗ transition is seen in which compound ?
A. Alcohol
B. Aromatic compound
C. Carbonyl compound
D. None of this
5. In uv SPECTROSCOPY water show peak on which λmax ?
A. 191 nm
B. 300 nm
C. 400 nm
D. 500 nm
6. Carbonyl compound shows Which transition
A. π⇒π∗
B.σ⇒σ∗
C.n⇒σ∗
D.n⇒π∗
7. Aromatic compound shows which transition
A. π⇒π∗
B.σ⇒σ∗
C.n⇒σ∗
D.n⇒π∗
8. During n⇒π∗ show wave length is ?
A. More than 200 nm
B. Less than 200 nm
C. A and B
D. All of the above
9. Which compound shows π⇒π∗ ?
A. Double and triplet bond
B. Aromatic compound
C. A and B
D. None of this
10. Hydrocarbon are seen transition is ?
A. π⇒π∗
B.σ⇒σ∗
C.n⇒σ∗
D.n⇒π∗
Answer key
1. A
2. B
3. C
4. A
5.A
6. D
7. A
8. A
9. C
10. B
Participate in Online FREE GPAT TEST: CLICK HERE