WOOD WARD FIESER RULE For UV Spectroscopy and Calculation of λmax of Organic Compounds
Woodward fieser rule :-
In 1945 Robert Burns wood ward gave certain rule for correlating λmax with molecular structure.
In 1959 Louis Frederick Fieser modified these rules with more experimental data and the modified rule is known as wood ward fischer rule.
According to the wood ward fischer rule the λmax of the molecule can be calculated by using a formula :-
λmax = Base value + ∑substituents- contributes + ∑ other contributes
Base value :- Each type of diene or triene system is having a certain fixed value at which absorption take place, this value is known as base value.
There are three set of rule
1. Wood ward fieser rule for conjugated diene and polyenes.
2. For unsaturated carbonyl compound
3. For aromatic compound or benzoyl compound.
1. Wood ward fieser rule for conjugated diene and polyenes :-
A. Homo annular diene :– cyclic diene having conjugated double bond in same ring.
B. Hetero annular diene :– cyclic diene having conjugated double bond in different ring.
C. Endo cyclic double bond :– double bond present in a ring.
D. Exocyclic double bond :- double bond in which one of the double bond atom is a part of a ring.
E. Double bond extending :– when more double bond are present other than conjugated.
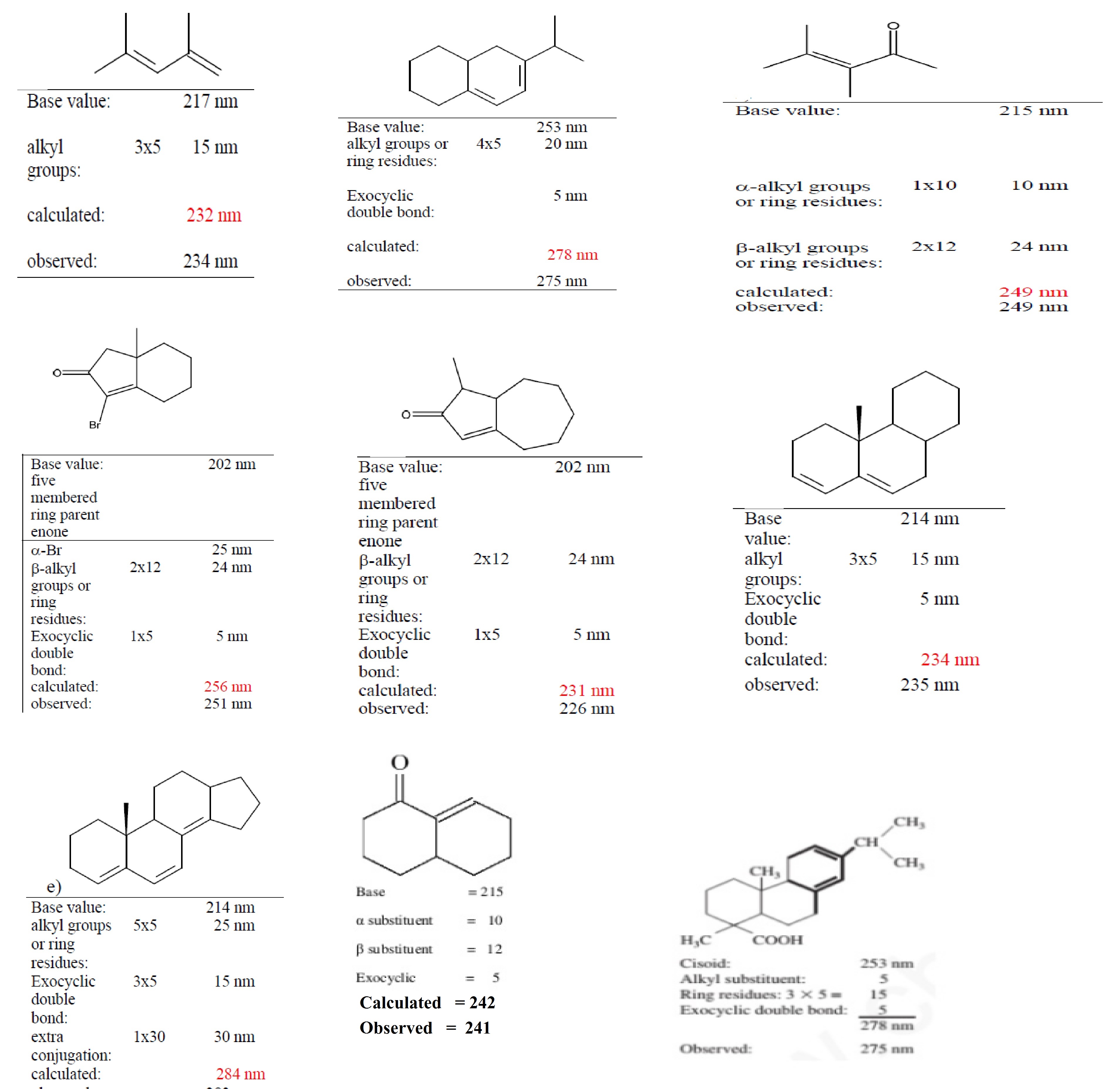
Different base value :-
Acyclic conjugated diene or Hetero annular conjugated diene has 215 nm wavelength.
Homo annular conjugated diene :-253 nm
Acyclic trienes :- 245 nm.
Each alkyl substitution , ring residue or ring residue :- 5 nm
Double bond extending conjugation :- 30 nm
Auxochrome base value :-
– OR = 6 nm
– SR = 30 nm
– CL,-Br = 5 nm
-OCOCH3 = 0nm
II) α, β UNSATURATED CARBONYL COMPOUNDS OR KETONES:
1. Base value: a) Acyclic α, β unsaturated ketones = 214 nm
b) 6 membered cyclic α, β unsaturated ketones = 215 nm
c) 5 membered cyclic α, β unsaturated ketones = 202 nm
d) α, β unsaturated aldehydes = 210 nm
e) α, β unsaturated carboxylic acids & esters = 195 nm
2. Alkyl substituent or Ring residue in α position = 10 nm
3. Alkyl substituent or Ring residue in β position = 12 nm
4. Alkyl substituent or Ring residue in γ and higher positions = 18 nm
5. Double bond extending conjugation = 30 nm
6. Exocyclic double bonds = 5 nm
7. Homodiene compound = 39 nm
8. Polar groups: a) –OH in α position = 35 nm
–OH in β position = 30 nm
–OH in δ position = 50 nm
b) –OAc in α, β, γ, δ positions = 6 nm
c) –OMe in α position = 35 nm
–OMe in β position = 30 nm
–OMe in γ position = 17 nm
–OMe in δ position = 31 nm
d) –Cl in α position = 15 nm
–Cl in β position = 12 nm
e) –Br in α position = 25 nm
–Br in β position = 30 nm
f) –NR2 in β position = 95 nm
III) AROMATIC COMPOUNDS:
1) Base value: for a) ArCOR = 246 nm
b) ArCHO = 250 nm
c) ArCO2H = 230 nm
d) ArCO2R = 230 nm
2) Alkyl group or ring residue in ortho and meta position = 3 nm
3) Alkyl group or ring residue in para position =10 nm
4) Polar groups: a) –OH, –OCH3, –OAlkyl in o, m position = 7 nm
b) –OH, –OCH3, –OAlkyl p position = 25 nm
c) –O (oxonium) in o position = 11 nm
d) –O (oxonium) in m position = 20 nm
e) –O (oxonium) in p position = 78 nm
f) –Cl in o, m position = 0 nm
g) –Cl in p position = 10 nm
h) –Br in o, m position = 2 nm
i) –Br in p position = 15 nm
j) –NH2 in o, m position = 13 nm
k) –NH2 in p position = 58 nm
l) –NHCOCH3 in o, m position = 20 nm
m) –NHCOCH3 in p position = 45 nm
n) –NHCH3 in p position = 73 nm
o) –N(CH3)2 in o, m position = 20 nm
p) –N(CH3)2 in p position = 85 nm
SAMPLE PROBLEMS
Participate in Online FREE GPAT TEST: CLICK HERE