BETHANECHOL Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
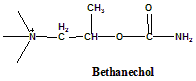
Bethanechol
IUPAC nomenclature
2-(Carbamoyloxy)-N,N,N-trimethylpropan-1-aminium.
Classification
Bethanechol is a cholinergic agonist falls in the class of choline esters.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 161.22 g/mol |
| 2 | Physical appearance | Present in solid form |
| 3 | Melting point | 220°C |
| 4 | Solubility | 10 mg/ml in chloride salt |
| 5 | Presence of ring | Not present |
| 6 | Number of chiral centers | 1 |
Mechanism of Action
- Bethanechol directly stimulates cholinergic receptors present in the parasympathetic nervous system.
- It stimulates the ganglia to a lower extent.
- It is having effect only on the muscarinic receptors. [1]
Structure Activity Relationship
- When the nitrogen of the quaternary ammonium group is replaced with arsenic, phosphorous, sulfur or selenium, their activity decreases.
- It is necessary for the atom present at the nitrogen position to have a positive charge to have muscarinic activity.
- When all three methyl groups are replaced by larger alkyl groups at the quaternary nitrogen, drug becomes inactive as agonist.
- When all the methyl groups associated with the quaternary nitrogen atom are replaced by ethyl groups, the compound becomes cholinergic antagonist.
- Replacement of just one methyl group associated with quaternary nitrogen with an ethyl or a propyl group gives the compound which is having lesser activity than acetylcholine.
- Substitution of the methyl groups associated with quaternary nitrogen with hydrogen atoms also leads to diminishing of the muscarinic activity.
- At the ethylene bridge, synthesis of acetic acid esters of quaternary ammonium alcohols of greater length than choline led to a series of compounds which are having lesser activity as the length of the chain increases.
- There should be no more than 5 atoms between nitrogen and terminal hydrogen to have the maximum muscarinic activity.
- Replacement of the ethylene bridge by the alkyl groups larger than methyl groups decreases the activity.
- The R-(-) isomer is 20 time less potent.
- For the maximum potency, the alkyl groups at the nitrogen atoms should not be larger than methyl groups.
- Presence of oxygen in an ester form increases the activity.
- For maximum activity, there should be two- carbon atoms between the nitrogen and oxygen atom. [2]
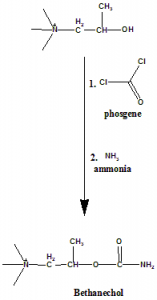
Method of synthesis
i. 1-(N,N,N-trimethylammonium)propan-2-ol is reacted with phosgene.
ii. Reaction is followed by addition of ammonia to give bethanechol. [3]
Therapeutic Uses
Bethanechol is used for treatment of:
- Urinary retention
Side Effects
Side effects of bethanechol are:
- Allergic reactions
- Shortness of breath
- Dizziness
- Headache
- Nausea
- Vomiting
- Irregular heart beats
- Sweating
- Tearing eyes
MCQs
Q.1 Match the following with correct SAR of the drug bethanchol-
| i. Nitrogen replaced with Arsenic | A. Muscarinic activity diminishes |
| ii. Introduction of the negative charge at Nitrogen position | B. Decreases activity |
| iii. Replacement of the Methyl groups with larger alkyl groups at Quaternary nitrogen | C. Activity decreases |
| iv. Replacement of the ethylene bridge with propyl group | D. Inactive as agonist |
a) i-A, ii-B, iii-C, iv-D
b) i-C, ii-A, iii-D, iv-B
c) i-D, ii-A, iii-B, iv-C
d) i-B, ii-C, iii-D, iv-A
Q.2 Correct sequence for the True/False for correct IUPAC names of the drug can be?
- Bethanchol: 2-(Carbamoyloxy)-N,N,N-trimethylpropan-1-aminium.
- Glycopyrollate: (–)-(S)-3-Hydroxy-2-phenylpropionic acid (1R,2R,4S,5S,7α,9S)-9-methyl-3-oxa-9-azatricyclo[3.3.1.02,4]non-7-yl ester
- Scopolamine: (1,1-dimethylpyrrolidin-1-ium-3-yl) 2-cyclopentyl-2-hydroxy-2-phenylacetate
- Propranolol: (RS)-1-(1-methylethylamino)-3-(1-naphthyloxy)propan-2-ol.
a) FTTF
b) FFFT
c) TFFT
d) TTTT
Q.3 Molecular weight of Bethanchol is?
a) 161.22 gm/mol
b) 225.3 gm/mol
c) 264.85gm/mol
d) 145.9 gm/mol
Q.4 Bethanchol produces effect on?
a) Nicotinic receptors
b) Muscarinic receptors
c) Both nicotinic and muscarinic receptors
d) None of the above
Q.5 Which amongst the following is a therapeutic use of drug bethanchol?
a) Treatment of peptic ulcers
b) Treatment of Blurred vision
c) Treatment of urinary retention
d) None of the above
Q.6 Which of the following drug and their classification are correct?
I. Bethanchol: Cholinergic agonist
II. Glycopyrollate: Acetylcholine antagonist
III. Scopolamine: Nicotinic receptors antagonist
IV. Propranolol: α-adrenergic antagonist
a) I , III
b) I , IV
c) III
d) I, II
Q.7 When compound ‘x’ reacts with 1-N,N,N-trimethylammonium)propan-2-ol, and on following the reaction by addition of ammonia, it produces Bethanchol.
Compound ‘x’ is?
a) Phosgene
b) Tricarboxylic acid
c) Phenol
d) Phenanthrene
FREE GPAT online Test: Participate: Click Here
ANSWERS
1-b
2-c
3-a
4-b
5-c
6-d
7-a
REFERENCES
[1] Downie JW. Bethanechol chloride in urology—a discussion of issues. Neurourology and Urodynamics. 1984;3(4):211-22. [2] Lemke TL, Williams DA, Foye WO. Principles of medicinal chemistry. Williams & Wilkins; 2017, 320. [3] Vardanyan R, Hruby V. Synthesis of essential drugs. Elsevier; 2006 Mar 10.