CHLORPROTHIXENE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
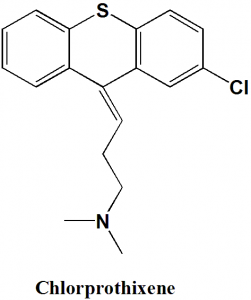
Chlorprothixene
IUPAC nomenclature
(Z)-3-(2-chlorothioxanthen-9-ylidene)-N,N-dimethyl-propan-1-amine.
Classification
Chlorprothixene is a thioxanthene antipsychotic drug.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 315.9 g/mol |
| 2 | Physical appearance | Pale yellow crystals |
| 3 | Melting point | 97-98°C |
| 4 | Solubility | Practically insoluble in water |
| 5 | Octanol/water partition coefficient | 5.18 |
| 6 | Presence of ring | Thioxanthene |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
- Chlorprothixene blocks postsynaptic dopaminergic D1 and D2 receptors in brain.
- Chlorprothixene also blocks α-adrenergic effects
- Depresses the release of hypothalamic and hypophyseal hormones
- Depresses the reticular activating system
- Due to these, basal metabolism, temperature, wakefulness, vasomotor tone and emesis of the body is affected.
Structure Activity Relationship
Structure activity relationship of phenothiazine like compounds can be described as follows:
- Tilting of side chain towards ring A grants favorable Vander Waal’s interaction of the side chain. This interaction decides the potency of the drug towards the dopamine receptors.
- Optimal neuroleptic activity occurs when the ring A substituent is in the 2nd-position.
- A trifluoromethyl substituent provides a greater number of favorable Van der Waal’s contacts with the side chain than the chlorine substituent. Thus, phenothiazne with trifluoromethyl substituents are more potent than those with chlorine substituent.
- A piperazine side chain provides more Van der Waal’s contacts with 2-substituent than the alkylamino side chain. Thus, piperizine phenothiazine are more potent in antischizophrenic effects than alkylamino phenothiazines.
- Hydroxyethylpiperazine side chain phenothiazines displays more favorable Van der Waal’s interactions with ring A than simple piperazines.
- In the thioxanthene and xanthenes containing ring systems, the cis forms are more potent neuroleptics than the trans isomers.
- Phenothiazine analogues having the presence of exolytic double bond are more potent than the corresponding compounds lacking the exolytic double bonds. [1]
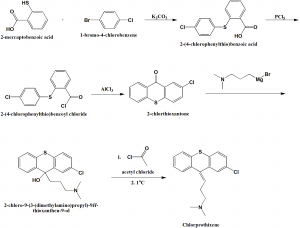
Method of synthesis
i. 2-mercaptobenzoic acid is reacted with 1-bromo-4-chlorobenzene to form 2-(4-chlorophenylthio)benzoic acid.
ii. The above formed compound is reacted with phosphorous pentachloride to transform the former into acid chloride.
iii. By using aluminum chloride, the compound undergoes intramolecular cyclization to produce 2-chlorthioxantone.
iv. 2-chlorthoxantone is reacted as carbonyl component with 3-dimethylaminopropylmagnesiumbromide to give tertiary alcohol.
v. Dehydration by acylation of the tertiary hydroxyl group using acetyl chloride and subsequent pyrolysis of the formed acetate produces chlorprothixene.
Therapeutic Uses
Chlorprothixene is used for:
- Treatment of schizophrenia
- Treatment of acute mania
- Treatment of anxiety
- Treatment of insomnia
- Controlling emesis and nausea
- Treatment of non-psychotic irritability
- Reducing aggression
Side Effects
Side effects of chlorprothixene are:
- Anticholinergic side effects
- Allergic side effects
- Liver damage
- Extrapyramidal side effects
MCQs
Q.1 Complete the following sentence related with the SAR of drug chlorprothixene:
“A piperazine side chain provides __________ Van der Waal’s contacts with 2-substituent than the alkylamino side chain. Thus, piperizine phenothiazine are __________ potent in antischizophrenic effects than alkylamino phenothiazines.”
a) More; more
b) More; less
c) Less; more
d) Less; less
Q.2 Correct sequence for the True/False for correct IUPAC names of the drug can be?
- Chlorprothixene: (Z)-3-(2-chlorothioxanthen-9-ylidene)-N,N-dimethyl-propan-1-amine
- Thioridazine: 10-[2-(1-methylpiperidin-2-yl)ethyl]-2-methylsulfanylphenothiazine
- Phenobarbital: 5,5-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione
- Secobarbital: 5-ethyl-5-pentan-2-yl-2-sulfanylidene-1,3-diazinane-4,6-dione
a) FFTF
b) TTFF
c) FTTF
d) FFFT
Q.3 Correct statement related with the solubility of Chlorprothixene is?
a) Practically insoluble in water
b) Slightly soluble in water
c) Soluble in water
d) Freely soluble in water
Q.4 Pick the correct statement?
a) Chlorprothixene stimulates postsynaptic dopaminergic D1 receptors only
b) Chlorprothixene stimulates postsynaptic dopaminergic D2 receptors only
c) Chlorprothixene stimulates postsynaptic dopaminergic D1 and D2 receptors
d) Chlorprothixene blocks postsynaptic dopaminergic D1 and D2 receptors
Q.5 Which amongst the following is a therapeutic use of drug Chlorprothixene?
a) Treatment of Schizophrenia
b) Treatment of Acute mania
c) Reducing aggression
d) All of the above
Q.6 Which of the following drug and their classification are correct?
I. Chlorprothixene: Phenothiazine antipsychotic drug
II. Tiotixene: Thioxanthene antipsychotic drug
III. Molindone: Nitrogen mustard
IV. Thiopental: barbiturate sedative hypnotic
a) I, II, IV
b) II, IV
c) III, IV
d) I, II, IV
Q.7 Number of chiral carbons present in the structure of chlorprothixene
a) 1
b) 2
c) 3
d) 0
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-a
2-b
3-a
4-d
5-d
6-b
7-d