DIMENHYDRINATE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Dimenhydrinate
Chemical composition
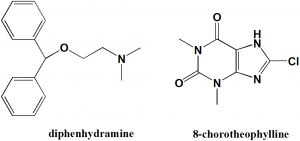
Diphenhydramine (53-55.5%) + 8-Chlorotheophylline (44-47%)
IUPAC nomenclature
2-benzhydryloxy-N,N-dimethylethanamine;8-chloro-1,3-dimethyl-7H-purine-2,6-dione
Classification
- H1-receptor antihistamine
- Ethanolamine ether antihistamine
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 470 g/mol |
| 2 | Physical appearance | Crystalline white powder |
| 3 | Melting point | 102-107oC |
| 4 | Solubility | Freely soluble in alcohol, soluble in water; insoluble in ether |
| 5 | Octanol/water partition coefficient | -0.39 |
| 6 | Presence of ring | Phenyl, purine |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
- The antiemetic property is due to H1 antagonism in the vestibular system.
- Excitatory effects of drug is due to adenosine receptor blockade by 8-chlorotheophylline.
Structure Activity Relationship
Structure activity of ethanolamine ethers can be summarized as:
- 8-chlorotheophyllinate salt of diphenhydramine is used for treatment of motion sickness
- Compounds with p-Cl-Ph and 2-pyridyl aryl groups are carboxamine, which is a potent anti-histamine drug.
- Substitution of the methyl group at the carbon alpha to the ether function gives a related compound known as doxylamine.
- An additional carbon atom between oxygen and nitrogen produces clemastine which has lower sedative properties.
- In setastine, alkyl amine substituent is incorporated into seven-membered hexahydroazepine ring, having lower sedative properties.
- With increase in alkyl group size at C-2’, there is decrease in antihistaminic activity and increases in anticholinergic activity..
- Introduction of alkyl substituents at C-4’results in decrease in anticholinergic activity and increase in antihistaminic activity. [1]
Method of synthesis
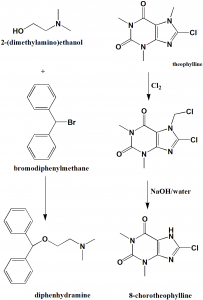
i. Reaction of benzhydrylbromide and 2-dimethylaminoethanol produces diphenhydramine.
ii. Theophylline anhydrous is warmed up to 50-60oC and dissolved in water, drip N-chlorosuccimide to get a crude product. The product is then dissolved in 5% NaOH aqueous solution to get Chlorotheophyline. [2]
Medicinal Uses
Dimenhydrinate is used for treatment of:
- Nausea
- Vomiting
- Dizziness
- Motion sickness
Side Effects
Side effects of dimenhydrinate are:
- Asthma
- Bronchitis
- Emphysema
- Glaucoma
- Liver diseases
- Kidney diseases
- Enlarged prostate
- Urination problems
- High blood pressure
- Overactive thyroid
- Heart diseases.
MCQs
Q.1 Dimenhydrinate is the mixture of?
a) Diphenhydramine and Epinephrine
b) Xylometazoline and Bicalutamide
c) Diphenhydramine and 8-chlorotheophylline
d) 8-chlorotheophylline and brompheniramine
Q.2 Which amongst the following statements is/are incorrect related to the SAR of ethanolamine ethers antihistamine drugs?
I. 8-chlorotheophyllinate salt of diphenhydramine is used for treatment of motion sickness
II. Compounds with p-Cl-Ph and 2-pyridyl aryl groups are carboxamine, which is a potent anti-histamine drug.
III. Substitution of the methyl group at the carbon alpha to the ether function gives a related compound known as doxylamine.
IV. An additional carbon atom between oxygen and nitrogen produces clemastine which has higher sedative properties.
a) I, II
b) II, IV
c) I, II, IV
d) IV
Q.3 Type of rings present in the structure of dimenhydrinate?
I. Pyridine
II. Purine
III. Phenyl
IV. Pyrimidine
a) II, III
b) I, IV
c) I, III
d) II, IV
Q.4 Side effects of drug dimenhydrinate is/are?
a) Asthma
b) Heart diseases
c) Enlarged prostate
d) All of the above
Q.5 Match the following drugs with their correct melting point-
| i. Dimenhydrinate | A. 211.5oC |
| ii. Epinephrine | B. 181.21oC |
| iii. Pyridostigmine | C. 282.22oC |
| iv. Niflumic acid | D. 102-107oC |
a. i-A, ii-D, iii-C, iv-B
b. i-D, ii-A, iii-B, iv-C
c. i-C, ii-B, iii-A, iv-D
d. i-B, ii-C, iii-A, iv-D
Q.6 An example of drug from class ethanolamine ether antihistamines is?
a) Dimenhydrinate
b) Dobutamine
c) Ethosuximide
d) All of the above
Q.7 Dimenhydrinate is used for the treatment of?
a) Nausea
b) Vomiting
c) Dizziness
d) All of the above
List of Successful GPATINDIAN Candidates
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-c
2-d
3-a
4-d
5-b
6-a
7-d
REFERENCES
[1] Lemke TL, Williams DA, editors. Foye’s principles of medicinal chemistry. Lippincott Williams & Wilkins; 2012 Jan 24. [2] Vardanyan R, Hruby V. Synthesis of essential drugs. Elsevier; 2006 Mar 10.