BROMODIPHENHYDRAMINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
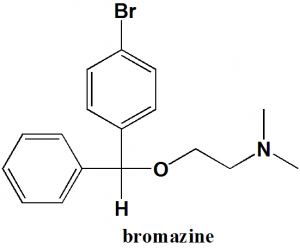
Bromazine
Or
Bromodiphenhydramine
IUPAC nomenclature
2-[(4-Bromophenyl)-phenylmethoxy]-N,N-dimethylethanamine
Classification
- H1-receptor antihistamine
- Ethanolamine ether antihistamine
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 334.2 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | 128-134oC |
| 4 | Solubility | 3.45e-03 g/L |
| 5 | Octanol/water partition coefficient | 4 |
| 6 | Presence of ring | Phenyl |
| 7 | Number of chiral centers | 1 |
Mechanism of Action
i. The drug binds with Histamine H1 receptor and produces antagonistic effects.
ii. This results in blockage of action of endogenous histamine
iii. Temporary relief of negative symptoms produced due to histamine
Structure Activity Relationship
Structure activity of ethanolamine ethers can be summarized as:
- 8-chlorotheophyllinate salt of diphenhydramine is used for treatment of motion sickness
- Compounds with p-Cl-Ph and 2-pyridyl aryl groups are carboxamine, which is a potent anti-histamine drug.
- Substitution of the methyl group at the carbon alpha to the ether function gives a related compound known as doxylamine.
- An additional carbon atom between oxygen and nitrogen produces clemastine which has lower sedative properties.
- In setastine, alkyl amine substituent is incorporated into seven-membered hexahydroazepine ring, having lower sedative properties.
- With increase in alkyl group size at C-2’, there is decrease in antihistaminic activity and increases in anticholinergic activity..
- Introduction of alkyl substituents at C-4’results in decrease in anticholinergic activity and increase in antihistaminic activity.[1]
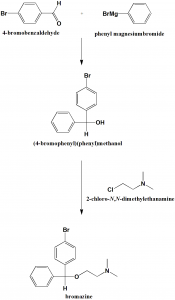
Method of synthesis
i. Reaction of 4-bromobenzaldehyde with phenyl magnesiumbromide to give (4-bromophenyl)(phenyl)methanol.
ii. (4-bromophenyl)(phenyl)methanol is reacted with 2-chloro-N,N-dimethylethanamine to give bromazine.
Medicinal Uses
Bromazine is used for treatment of:
- Allergic symptoms
- Irritant cough
- Nausea
- Vomiting
- Vertigo
- Motion sickness
- Drug-induced extrapyramidal symptoms
- Mild symptoms of Parkinson’s disease
Side Effects
Side effects of bromazine are:
- Drowsiness
- Blurred vision
- Restlessness
- Dizziness
- Headache
- Stomach upset
- Constipation
- Dry mouth, nose and throat
- Difficulty in breathing
- Mental or mood changes
- Hallucinations
- Allergic reactions
- Seizures
MCQs
Q.1 Bromazine produces its effect through?
a) Binding with H1 receptors and producing agonizing effects
b) Binding with H1 receptors and producing antagonizing effects
c) Binding with H2 receptors and producing agonizing effects
d) Binding with H2 receptors and producing antagonizing effects
Q.2 Therapeutic use of drug bromazine is/are?
a) Treatment of allergic symptoms
b) Treatment of nausea
c) Treatment of motion sickness
d) All of the above
Q.3 Which amongst the following are the correct statements with respect to the SAR of ethanolamine ethers antihistamine drugs?
I. Compounds with p-Cl-Ph and 2-pyridyl aryl groups are carboxamine, which is a potent anti-histamine drug.
II. Substitution of the methyl group at the carbon alpha to he ether function gives a related compound known as doxylamine.
III. An additional carbon atom between oxygen and nitrogen produces clemastine which has lower sedative properties.
IV. In setastine, alkyl amine substituent is incorporated into seven-membered hexahydroazepine ring, having lower sedative properties.
a) I , II, III, IV
b) II, IV
c) II, III
d) I, IV
Q.4 The starting chemicals required for the synthesis of drug bromazine are?
a) 4-bromobenzadehyde and phenyl magnesiumbromide
b) 4-chlorobenzaldehyde and phenyl magnesiumchloride
c) Bromobenzene and Ethanol
d) Naphthol and Antherenol
Q.5 Correct sequence for the True/False for the physiochemical properties of the drug Bromazine can be?
I. Molecular weight:200.32 gm/mol
II. Melting point:128-134oC
III. Octanol/water partition coefficient:4
a) FTF
b) FTT
c) FFT
d) TTT
Q.6 Correct IUPAC nomenclature of drug bromphenhydramine can be?
a) 2-[(4-chlorophenyl)-phenylmethoxy]-N,N-dimethylethanamine
b) 2-Bromo-2-chloro-1,1,1-trifluoropropane
c) 2-[(4-Bromophenyl)-phenylmethoxy]-N,N-dimethylethanamine
d) 2-Bromo-2-chloro-1,1,1-trifluoroethane
Q.7 Match the following drugs with the correct number of chiral carbons they have in their structure:
| i. Bromphenhydramine | A. 0 |
| ii. Gabapentin | B. 3 |
| iii. Benztropin | C. 7 |
| iv. Finasteride | D. 1 |
a. i-A, ii-D, iii-B, iv-C
b. i-C, ii-B, iii-D, iv-A
c. i-C, ii-A, iii-D, iv-B
d. i-D, ii-A, iii-B, iv-C
List of Successful GPATINDIAN Candidates
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-b
2-d
3-a
4-a
5-b
6-c
7-d
REFERENCES
[1] Lemke TL, Williams DA, editors. Foye’s principles of medicinal chemistry. Lippincott Williams & Wilkins; 2012 Jan 24.