CARBACHOL Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
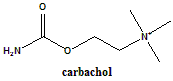
Carbachol
IUPAC nomenclature
2-[(Aminocarbonyl)oxy]-N,N,N-trimethylethanaminium chloride.
Classification
Carbachol is a cholinergic agonist falls in the class of choline esters.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 182.65 g/mol |
| 2 | Physical appearance | It is present in hard prismatic crystal form or as powder. |
| 3 | Melting point | 200-203°C |
| 4 | Solubility | 1gm in 1ml of water |
| 5 | Presence of ring | Not present |
| 6 | Number of chiral centers | Not present |
Mechanism of Action
- Carbachol stimulates both muscarinic and nicotinic receptors through parasympathomimetic action.
- When it is used in topical ocular or intraocular, it produces effects like miosis and increased aqueous humor outflow.
Structure Activity Relationship
- When the nitrogen of the quaternary ammonium group is replaced with arsenic, phosphorous, sulfur or selenium, their activity decreases.
- It is necessary for the atom present at the nitrogen position to have a positive charge to have muscarinic activity.
- When all three methyl groups are replaced by larger alkyl groups at the quaternary nitrogen, drug becomes inactive as agonist.
- When all the methyl groups associated with the quaternary nitrogen atom are replaced by ethyl groups, the compound becomes cholinergic antagonist.
- Replacement of just one methyl group associated with quaternary nitrogen with an ethyl or a propyl group gives the compound which is having lesser activity than acetylcholine.
- Substitution of the methyl groups associated with quaternary nitrogen with hydrogen atoms also leads to diminishing of the muscarinic activity.
- At the ethylene bridge, synthesis of acetic acid esters of quaternary ammonium alcohols of greater length than choline led to a series of compounds which are having lesser activity as the length of the chain increases.
- There should be no more than 5 atoms between nitrogen and terminal hydrogen to have the maximum muscarinic activity.
- Replacement of the ethylene bridge by the alkyl groups larger than methyl groups decreases the activity.
- The R-(-) isomer is 20 time less potent.
- For the maximum potency, the alkyl groups at the nitrogen atoms should not be larger than methyl groups.
- Presence of oxygen in an ester form increases the activity.
- For maximum activity, there should be two- carbon atoms between the nitrogen and oxygen atom. [1]
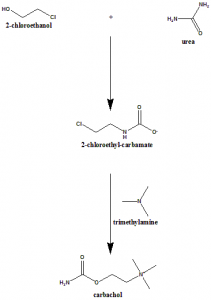
Method of synthesis
The interaction of beta-chloroethylcarbamate and trimethylamine produces carbachol.
Therapeutic Uses
Carbachol is used for treatment of:
- Glaucoma
Side Effects
Side effects of carbachol are:
- Seeing floaters in the vision
- Irregular heart beats
- Trouble breathing
- Headache
- Stomach cramps
- Diarrhea
- Eye irritation
- Increased sweating and urination
MCQs
Q.1 What can be the correct IUPAC nomenclature of carbachol?
a) (RS)-1-(1-methylethylamino)-3-(1-naphthyloxy)propan-2-ol.
b) [8-methyl-8-(1-methylethyl)- 8-azoniabicyclo[3.2.1] oct-3-yl] 3-hydroxy-2-phenyl-propanoate
c) (RS)-(8-Methyl-8-azabicyclo[3.2.1]oct-3-yl) 3-hydroxy-2-phenylpropanoate
d) 2-[(Aminocarbonyl)oxy]-N,N,N-trimethylethanaminium chloride.
Q.2 Which amongst the following statements is/are incorrect related to the SAR of Carbachol?
I. For the maximum potency, the alkyl groups at the nitrogen atoms should not be larger than methyl groups.
II. Presence of oxygen in an ester form decreases the activity.
III. For maximum activity, there should be two- carbon atoms between the nitrogen and oxygen atom.
a) II
b) I
c) II, I
d) II, III
Q.3 The interaction of beta-chloroethylcarbamate and trimethylamine produces?
a) Atropin
b) Carbachol
c) Procyclidine
d) Doxacurium
Q.4 Side effects of drug Carbachol is/are?
a) Irregular heart beats
b) Trouble breathing
c) Diarrhea
d) All of the above
Q.5 Match the following drugs with correct number of chiral carbons they are having.
| i. Propranolol | A. 1 |
| ii. Carbachol | B. 4 |
| iii. Ipratropium bromide | C. 0 |
| iv. Atropine | D. 5 |
a) i-A, ii-C, iii-B, iv-D
b) i-D, ii-,B iii-A, iv-C
c) i-B, ii-D, iii-A, iv-C
d) i-A, ii-C, iii-D, iv-B
Q.6 An example of drug from class cholinergic agonist is?
a) Donepezil
b) Carbachol
c) Atropin
d) Paraoxin
Q.7 The type of ring system found in Carbachol?
a) Benzene
b) Napthalene
c) Imidazole
d) None of the above
FREE GPAT online Test: Participate: Click Here
ANSWERS
1-d
2-a
3-b
4-d
5-d
6-b
7-d
REFERENCES
[1] Lemke TL, Williams DA, Foye WO. Principles of medicinal chemistry. Williams & Wilkins; 2017, 320.