CHLORPROMAZINE HYDROCHLORIDE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
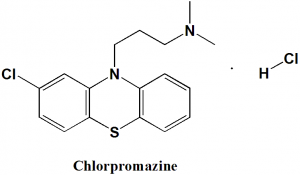
Chlorpromazine hydrochloride
IUPAC nomenclature
3-(2-chlorophenothiazin-10-yl)-N,N-dimethylpropan-1-amine;hydrochloride.
Classification
Chlorpromazine hydrochloride is phenothiazine antipsychotic drug.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 355.3 g/mol |
| 2 | Physical appearance | White or creamy white crystalline powder. |
| 3 | Melting point | 195°C |
| 4 | Solubility | 100 mg/ml |
| 5 | Octanol/water partition coefficient | N/A |
| 6 | Presence of ring | Phenothiazine |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
Chlorpromazine hydrochloride acts as antagonist for following postsynaptic receptors:
- Dopaminergic receptors subtypes D1, D2, D3 and D4, which results in antipsychotic properties on productive and unproductive symptoms.
- Serotonergic receptors 5-HT1 and 5-HT2, which is responsible for the anxiolytic, antidepressive and antiaggressive properties and also as an attenution of extrapyramidal side effects of the drug.
- Histaminergic receptors H1 subtype, which is responsible for sedation, antiemesis, vertigo, lowering of bloob pressure and weight gain properties of the drug.
- α1 and α2 receptors which are responsible for the properties of drug like antisympathomimetic, lowering of blood pressure, reflex tachycardia, Vertigo. Sedation. Hypersalivation and incontinence and also sexual dysfunctions; may also attenuate peudoparkinsonism.
- Muscarinic M1 and M2 receptors which causes anticholinergic symptoms like dry mouth and blurred vision.
- It is also a weak presynaptic inhibitor of dopamine reuptake which results in mild antidepressive and antiparkinsonian effects. The action could also account for psychomotor agitation and amplification of psychosis.
Structure Activity Relationship
Structure activity relationship of phenothiazine can be described as follows:
- Tilting of side chain towards ring A grants favorable Vander Waal’s interaction of the side chain. This interaction decides the potency of the drug towards the dopamine receptors.
- Optimal neuroleptic activity occurs when the ring A substituent is in the 2nd-position.
- A trifluoromethyl substituent provides a greater number of favorable Van der Waal’s contacts with the side chain than the chlorine substituent. Thus, phenothiazne with trifluoromethyl substituents are more potent than those with chlorine substituent.
- A piperazine side chain provides more Van der Waal’s contacts with 2-substituent than the alkylamino side chain. Thus, piperizine phenothiazine are more potent in antischizophrenic effects than alkylamino phenothiazines.
- Hydroxyethylpiperazine side chain phenothiazines display more favorable Van der Waal’s interactions with ring A than simple piperazines.
- In the thioxanthene and xanthenes containing ring systems, the cis forms are more potent neuroleptics than the trans isomers.
- Phenothiazine analogues having the presence of exolytic double bond are more potent than the corresponding compounds lacking the exolytic double bonds. [1]
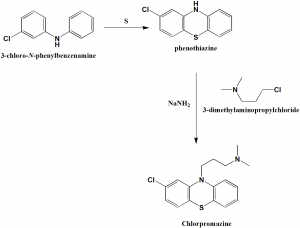
Method of synthesis
i. 3-chloro-N-phenylbenzenamine reacts with sulfur to give 2-chloro-10H-phenothiazine.
ii. Chlorpromazine can be synthesized from 2-chloro-10H-phenothiazineby alkylation with 3-dimethylaminopropylchloride in the presence of sodium amide. [2]
Therapeutic Uses
Chlorpromazine hydrochloride is used for:
- Treatment of schizophrenia
- Treatment of psychotic disorders
- Treatment of manic phase of bipolar disorder
- Treatment of severe behavior problems in children
- Lowering nervousness
- Reducing aggressive behavior
- Decreasing hallucinations
Side Effects
Side effects of Chlorpromazine hydrochloride are:
- Drowsiness
- Dizziness
- Lightheadedness
- Dry mouth
- Blurred vision
- Nausea
- Tiredness
- Trouble sleeping
- Weight gain
- Constipation
- Extrapyramidal symptoms
MCQs
Q.1 What can be the correct IUPAC nomenclature of chlorpromazine hydrochloride?
a) 4-[4-(4-Chlorophenyl)-4-hydroxypiperidin-1-yl]-1-(4-fluorophenyl)butan-1-one;hydrochloride
b) 10-[3-(4-methylpiperazin-1-yl)propyl]- 2-(trifluoromethyl)-10H-phenothiazine;hydrochloride
c) 3-(2-chlorophenothiazin-10-yl)-N,N-dimethylpropan-1-amine;hydrochloride
d) N,N-dimethyl-3-phenothiazin-10-ylpropan-1-amine;hydrochloride
Q.2 Which amongst the following statements is/are INCORRECT related to the SAR of chlorpromazine hydrochloride?
I. Optimal neuroleptic activity occurs when the ring A substituent is in the 3rd-position.
II. A trifluoromethyl substituent pro, vides a greater number of favorable Van der Waal’s contacts with the side chain than the chlorine substituent. Thus, phenothiazne with trifluoromethyl substituents are more potent than those with chlorine substituent.
III. A piperazine side chain provides more Van der Waal’s contacts with 2-substituent than the alkylamino side chain. Thus, piperizine phenothiazine are less potent in antischizophrenic effects than alkylamino phenothiazines.
IV. Hydroxyethylpiperazine side chain phenothiazines displays more favorable Van der Waal’s interactions with ring A than simple piperazines.
a) I, III, IV
b) I, II
c) III, IV
d) I, III
Q.3 The correct order for the synthesis of drug Chlorpromazine hydrochloride from 3-chloro-N-phenylbenzenamine can be?
I. Reaction with Iodine
II. Reaction with sulfur
III. Alkylation with 3-dimethylaminopropylchloride
IV. Alkylation with 2-methylaminopropylchloride
a) I – III
b) I – IV
c) II – III
d) II – IV
Q.4 Side effects of drug Chlorpromazine hydrochloride is/are?
a) Dizziness
b) Drowsiness
c) Extrapyramidal symptoms
d) All of the above
Q.5 Match the following drugs with their correct melting points:
| i. Chlorpromazine hydrochloride | A. 145.75oC |
| ii. Droperidol | B. 200oC |
| iii. Atazanavir | C. 195oC |
| iv. Thiamylal | D. 133.5 oC |
a) i-A, ii-C, iii-B, iv-D
b) i-C, ii-A, iii-D, iv-B
c) i-C, ii-A, iii-B, iv-D
d) i-B, ii-D, iii-A, iv-C
Q.6 An example of drug from class phenothiazine antipsychotic drug is?
a) Tacrine
b) Chlorpromazine
c) Procyclidine
d) Parathion
Q.7 The type of ring system found in Chlorpromazine hydrochloride?
a) Phenothiazine
b) Piperizine ring
c) Pyrimidine ring
d) Pyridine ring
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-c
2-d
3-c
4-d
5-c
6-b
7-a