CISPLATIN Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
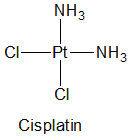
Cisplatin
IUPAC nomenclature
(SP-4-2)-diamminedichloroplatinum(II)
Classification
Cisplatin falls under the category of Antineoplastic cytotoxic drug. [1]
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 300 g/mol |
| 2 | Appearance | Platinol vials contains cisplatin as a lyophilized powder form. |
| 3 | Melting point | 270°C |
| 4 | Solubility | Solubility in water is 2.53g/L |
| 5 | Presence of ring | No ring structure present |
Mechanism of Action
i. It introduces the alkyl groups to the DNA. When repair enzymes tries to remove the alkylaed fragments, they fragment the DNA.
ii. It binds with DNA and produces cross-linkages.
iii. It induces mispairing of the DNA causes mutations in the cells.[2]
Structural Activity Relationship
- Cytotoxicity of oxiplatin is also greater than cisplatin.
- Organic functionalities of nonleaving groups coordinated to platinum are critical for selective uptake by OTCs. [3]
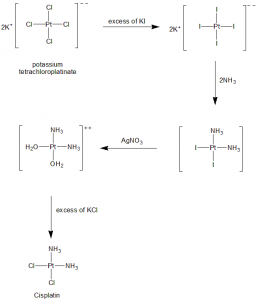
Methods of Synthesis
i. Potassium tetrachloroplatinate is reacted with excess of potassium iodide.
ii. The tetraiodide so formed is reacted with ammonia to form K2[PtI2(NH3)2], a yellow compound.
iii. The insoluble silver iodide is precipitated as the yellow compound is treated with silver nitrate in water.
iv. Addition of the potassium chloride gives the final product.
Therapeutic Uses
Cisplatin is given for the treatment for :
- Mesothelioma
- Melanoma
- Multiple myeloma
- Sarcomas
- Neuroblastoma
- Hodgkin’s lymphomas
- Non-Hodgkin’s lymphoma
- Prostate cancer
- Stomach cancer
- Cerviacal cancer
- Breast cancer
- Small cell lung cancer
- Non-small cell lung cancer
- Esophageal cancer
- Head and neck cancer
- Bladder cancer
- Ovarian cancer
- Testicular cancer
- Metastatic cancer
- Metastatic ovarian cancer
- Advanced bladder cancer
Side Effects
- Common side effects of cisplatin include nausea, vomiting, low blood counts, kidney toxicity, ototxicity and blood test abnormalities.
- Less common side effects of cispatin include peripheral neuropathy, loss of appetite, taste changes, increase in blood tests measuring liver function and hair loss.
MCQs
Q.1 Match the following with correct IUPAC nomenclatures
| i. (SP-4-2)-diamminedichloroplatinum(II) | A. Carboplatin |
| ii. cis diammine(cyclobutane-1,1-dicarboxylate-O,O’)platinum(II)
|
B. Procarbazine |
| iii. N-Isopropyl-4-[(2-methylhydrazino)methyl]benzamide
|
C. Cisplatin |
a) i-A, ii-C, iii-B
b) i-B, ii-A, iii-C
c) i-C, ii-B, iii-A
d) i-C, ii-A, iii-B
Q.2 How many statements below are true with respect to the SAR of the drug cisplatin?
- Trans form is less cytotoxic than cis form.
- Cytotoxicity of oxiplatin is greater than cisplatin.
- Organic functionalities of nonleaving groups coordinated to platinum are critical for selective uptake by OTCs.
a) 1
b) 2
c) 3
d) 0
Q.3 The drug cisplatin is found in which form
a) Lyphophillized powder form
b) Hydrophillized powder form
c) Can be in either of lypopyhllized or hydrophyllized powder form
d) None of the above
Q.4 The number of incorrect statements with respect to the method of synthesis of the drug cisplatin is?
- Synthesis starts with potassiumtetrachloroplatinate.
- K2[PtI2(NH3)2 is a red colored compound.
- The final product is obtained after addition of potassium cyanide
a) 1
b) 2
c) 3
d) 0
Q.5 Which amongst the following is not a therapeutic use of drug cisplatin?
a) Down’s diseases
b) Hodgkin’s lymphomas
c) Non-Hodgkin’s lymphomas
d) Small-cell lung cancer
Q.6 The correct classification of the drug cisplatin can be?
a) Calcium channel blocker
b) Antiarrhythemic drug
c) Antineoplastic cytotoxicity drug
d) Immuosuppressive agent
Q.7 How many number of rings are found in the chemical structure of the drug cisplatin?
a) 0
b) 1
c) 2
d) 3
ANSWERS
1-d
2-b
3-a
4-a
5-a
6-c
7-a
REFERENCES
[1] Tripathi KD. Essentials of Medical Pharmacology, 6thEdn. Jaypee Brothers Medical Publishers (P) Ltd. 2008: 820. [2] Fuertes MA, Castilla J, Alonso C, Prez JM. Cisplatin biochemical mechanism of action: from cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Current medicinal chemistry. 2003 Feb 1;10(3):257-66.