DICLOFENAC Synthesis, SAR,MCQ,Structure,Chemical Properties and Therapeutic Uses
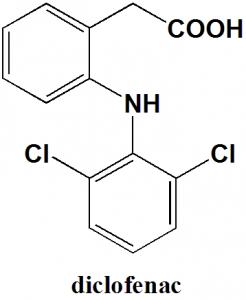
Diclofenac
IUPAC nomenclature
[2-(2,6-Dichloroanilino)phenyl]acetic acidClassification
- Nonsteroidal anti-inflammatory drug
- Phenyl acetic acid derivative
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 296.1 g/mol |
| 2 | Physical appearance | Crystals from ether-petroleum ether |
| 3 | Melting point | 283-285°C |
| 4 | Solubility | 2.73 mg/L in water |
| 5 | Octanol/water partition coefficient | 4.51 |
| 6 | Presence of ring | Phenyl |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
- Diclofenac inhibits cyclooxygenases (COX-1 and COX-2) which in turn decreases the production of prostaglandins.
- Prostaglandins are responsible for the transmission of pain and inflammation and thus, reducing the amount f prostaglandins helps in reducing the pain and inflammation in the body.
Structure Activity Relationship
SAR of Diclofenac can be summarized as follows: The two-o-chloro groups are important for the activity of the drug. the twisting of the two o-chloro groups is necessary for the binding of the drug with COX active site.
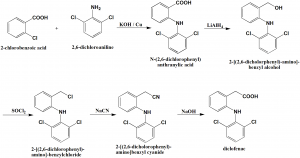
Method of synthesis
i. Reaction of 2-chlorobenzoic acid and 2,6-dichloroaniline in the presence of sodium hydroxide and copper produces N-(2,6-dichlorophneyl)anthranylic acid.
ii. The carboxylic group of the last compound is reduced by lithium aluminum hydride to give 2-[(2,6-dichlorophenyl)-amino]-benzyl alcohol.
iii. The above formed compound is further chlorinated by thionyl chloride to produce 2-[(2,6-dichlorophenyl)-amino]-benzylchloride.
iv. On further reaction with sodium cyanide, 2-[(2,6-dichlorophenyl)-amino]benzyl cyanide is formed.
v. On hydrolysis of the nitrile group of last compound leads to formation of diclofenac. [2]
Therapeutic Uses
Diclofenac is used for:
- Reducing pain, joint stiffness and swelling due to arthritis.
Side Effects
Side effects of Diclofenac are:
- Nausea
- Vomiting
- Heartburn
- Diarrhea
- Upset stomach
- Gas
- Dizziness
- Drowsiness
- Headache
- Hypertension
- Ringing in ears
- Mood changes
- Symptoms of heart failures
- Kidney problems
- Stiff neck
- Liver disease
- Allergic reactions
MCQs
Q.1 Choose the correct option related with the mechanism of action of drug Diclofenac?
a) Inhibits the activity of COX-1
b) Inhibits the activity of COX-2
c) Decreases in the production of prostaglandins
d) All of the above
Q.2 Medicinal use of drug Diclofenac is/are?
a) Reducing pain due to arthritis
b) Treatment of psoriases
c) Treatment of warts
d) All of the above
Q.3 Type of ring structure present in the Diclofenac is
a) Phenyl
b) Cyclohexane
c) Furan
d) Diazepine
Q.4 Number of chiral carbons present in the structure of diclofenac is?
a) 0
b) 1
c) 2
d) 3
Q.5 Correct sequence for the True/False for the physiochemical properties of the drug diclofenac is?
I. Molecular weight = 296.1gm/mol
II. Melting point = 147oC
III. Crystals from ether-petroleum ether
IV. Ring structure is absent
a) TFTF
b) TFFT
c) FTTF
d) TFFF
Q.6 Correct statements for the IUPAC nomenclatures of the drugs are?
I. Diclofenac:2-hydroxybenzoate
II. Levallorphan: 2-Chloro-N-(2-chloroethyl)-N-methylethan-1-amine
III. Dacarbazine: N,3-Bis(2-chloroethyl)-1,3,2-oxazaphosphinan-2-amide 2-oxide
IV. Carboplatin: (SP-4-2)-diamminedichloroplatinum(II)
a) I, IV
b) I, II, III, IV
c) II , III
d) None
Q.7 Match the following drugs with their correct classifications-
| i. Diclofenac | A. Inhalational anesthetics |
| ii. Bisoprolol | B. ß-adrenergic blocker |
| iii. Thioridazine | C. Nonsteroidal anti-inflammatory drug |
| iv. Enflurane | D. Phenothiazine antipsychotic drug |
a) i-B, ii-D, iii-C, iv-A
b) i-B, ii-C, iii-A, iv-D
c) i-C, ii-B, iii-D, iv-A
d) i-A, ii-D, iii-B, iv-C
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-d
2-a
3-a
4-a
5-a
6-d
7-c