DIHYDROERGOTAMINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
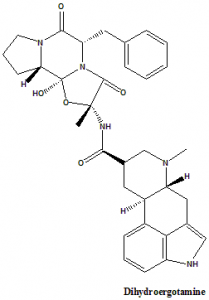
Dihydroergotamine
IUPAC nomenclature
(2R,4R,7R)-N-[(1S,2S,4R,7S)-7-benzyl-2-hydroxy-4-methyl-5,8-dioxo-3-oxa-6,9-diazatricyclo[7.3.0.02,6]dodecan-4-yl]-6-methyl-6,11-diazatetracyclo[7.6.1.02,7.012,16]hexadeca-1(16),9,12,14-tetraene-4-carboxamide.
Classification
Dihydroergotamine is an ergot alkaloid.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 583.7 g/mol |
| 2 | Physical appearance | Present in solid form. |
| 3 | Melting point | 219°C |
| 4 | Solubility | Water solubility is 0.229 mg/ml |
| 5 | Octanol/water partition coefficient | 2 |
| 6 | Presence of ring | Ergoline ring |
| 7 | Number of chiral centers | 7 |
Mechanism of Action
Dihydroergotamine is proposed to work on two theories:
- Activation of 5-HT10 receptors located on the intracranial blood vessels, which also includes the receptors on arterio-venous anastomoses. This results in vasoconstriction and thus, produces relief in migraine and headache.
- Inhibition of pro-inflammatory neuropeptide release through activation of 5-HT10 receptors on sensory nerve endings of the trigeminal system.
Structure Activity Relationship
- d-isomers of lysergic acid is inactive. It must be in the l-form.
- Antimigraine effect of the drug is due to the 9-10 double bond.
Method of synthesis
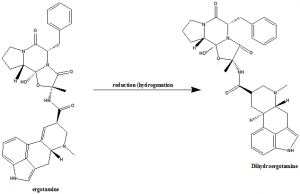
Dihydroergotamine can be synthesized from reduction (hydrogenation) of the double bond of the ergoline ring of ergotamine at the 9-0 position.
Therapeutic Uses
Dihydroergotamine is used for:
- Migraine headaches
- Cluster headaches
- Prevention of migrains
Side Effects
Side effects of Dihydroergotamine are:
- Increased sweating
- Diarrhea
- Vomiting
- Nausea
- Headache
- Drowsiness
- Dizziness
MCQs
Q.1 Mechanism of action of dihydroergotamine depends on?
a) Activation of 5-HT10 receptors located on the intracranial blood vessels
b) Inhibition of pro-inflammatory neuropeptide release through activation of 5-HT10 receptors on sensory nerve endings of the trigeminal system
c) Both a) and b)
d) None of the above
Q.2 Therapeutic use of drug dihydroergotamine is/are?
a) Migraine headaches
b) Cluster headaches
c) Prevention of migrains
d) All of the above
Q.3 Which amongst the following are the correct statements with respect to the SAR of drug dihydrergotamine?
I. d-isomers of lysergic acid is inactive. It must be in the l-form.
II. Antimigraine effect of the drug is due to the 9-10 double bond.
a) I, II
b) I
c) II
d) NONE
Q.4 Dihydroergotamine can be synthesized from ergotamine by?
a) Halogination
b) Reduction
c) Halogination followed by reduction
d) None of the above
Q.5 Correct sequence for the True/False for the physiochemical properties of the drug Dihydroergotamine?
I. Molecular weight is 222.3 gm/mol.
II. Melting point is 219°C
III. it is having an imidazole ring
IV. Number of chiral carbon atoms is 3
a) TTFF
b) TFTF
c) FFTF
d) TTTT
Q.6 Trade name of Dihdroergotamine is?
a) D.H.E. 45
b) Alkeran
c) Mustine
d) Endoxan
Q.7 Match the following drugs with their correct classifications-
| i. Bitolterol | A. ß2-ADRENERGIC AGONIST |
| ii. Pseudoephedrine | B. MIXED ACTING SYMPATHOMIMETICS |
| iii. Phenoxybenzamine | C. NONSELECTIVE α-ADRENERGIC ANTAGONIST |
| iv. Methyldopa | D. SELECTIVE α2-ADRENERGIC AGONIST |
a) i-A, ii-B, iii-C, iv-D
b) i-D, ii-B, iii-C, iv-A
c) i-C, ii-A, iii-B, iv-D
d) i-D, ii-B, iii-A, iv-C
ANSWERS
1-c
2-d
3-a
4-b
5-a
6-a
7-a