DIMETHINDENE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Dimethindene
IUPAC nomenclature
N,N-Dimethyl-2-[3-(1-pyridin-2-ylethyl)-1H-inden-2-yl]ethan-1-amine
Classification
- H1-receptor antihistamine
- Alkylamine antihistamine
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 292.4 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | 158oC |
| 4 | Solubility | 239 mg/LL |
| 5 | Octanol/water partition coefficient | N/A |
| 5 | Presence of ring | Phenyl, indene |
| 6 | Number of chiral centers | 1 |
Mechanism of Action
i. Dimethindene binds to histamine H1 receptors and blocks the action of endogenous histamine.
ii. This provides relief from the negative symptoms produced by the histamine.
Structure Activity Relationship
Structure activity of alkyl amines antihistamines can be summarized as:
- E- and Z- isomers in alkenes shows large difference in activity, where, E-isomers are more potent than Z-
- The two aromatic rings have different binding environments at the receptors.
- 5-6 angstrom distance is required between aromatic ring and tertiary aliphatic amine for biding at the receptor.
- S-enantiomers have greater affinity for H1 histamine receptors [1]
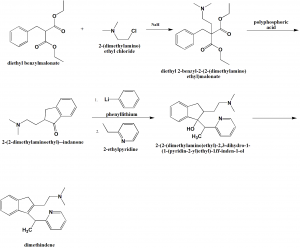
Method of synthesis
i Diethyl benzylmalonate is reacted with 2-(dimethylamino)ethyl chloride in presence of sodium to give diethyl 2-benzyl-2-(2-(dimethylamino) ethyl)malonate
ii. Above formed compound is reacted with polyphosphoric acid to give 2-(2-dimethylaminoethyl)—indanone.
iii. Last compound is reacted with phenyllithium and 2-ethylpyridine to form 2-(2-(dimethylamino)ethyl)-2,3-dihydro-1- (1-(pyridin-2-yl)ethyl)-1H-inden-1-ol.
iv. Above formed compound is converted into dimethindine by reduction [2]
Medicinal Uses
Dimethindene is used for treatment of:
- Allergies
- Hay fever
- Common cold
- Watery eyes
- Runny nose
- Sneezing
- Urticaria
- As an adjuvant in ecszema
Side Effects
Side effects of Dimethindene are:
- Seizures
- Loss of consciousness
- Hallucinations
- Drowsiness
- Dizziness
- Allergic reactions
- Confusion
- Nervousness
- Increased urination
- Blurred vision
- Dry mouth/throat/nose
- Restlessness
MCQs
Q.1 IUPAC nomenclature of drug dimethindene is?
a) N,N-Dimethyl-2-[3-(1-pyridin-2-ylethyl)-1H-inden-2-yl]ethan-1-amine
b) N,N-Dimethyl-3-phenyl-3-pyridin-2-yl-butan -1-amine
c) N,N-Dimethyl-2-[3-(1-pyridin-2-ylethyl)-1H-inden-2-yl]propan-1-amine
d) 2-[(4-Bromophenyl)-phenylmethoxy]-N,N-dimethylpropylamine
Q.2 Which amongst the following statements is/are incorrect related to the SAR of alkylamine antihistamine drugs?
I. E- and Z- isomers in alkenes shows large difference in activity, where, E-isomers are less potent than Z-isomers.
II. The two aromatic rings have different binding environments at the receptors.
III. 5-6 angstorm distance is required between aromatic ring and tertiary aliphatic amine for binding at the receptor.
IV. S-enantiomers have lesser affinity for H1 histamine receptors.
a) I, IV
b) II, IV
c) I, II, IV
d) IV
Q.3 Correct sequence for synthesis of drug dimethindene from diethyl benzylmalonate can be?
I. Reaction with phenyllithium and 2-ethylpyridine
II. Reaction with polyphosphoric acid
III. Reaction with 2-(dimethylamino)ethyl chloride
IV. Reduction
a) II – III – I – IV
b) III – II – I – IV
c) I – II – III – IV
d) II – IV – I – III
Q.4 Side effects of drug dimethindene is/are?
a) Dry mouth
b) Seizures
c) Hallucinations
d) All of the above
Q.5 Match the following drugs with their correct melting point-
| i. Dimethindene | A. 199oC |
| ii. Clemastine | B. 178oC |
| iii. Piroxicam | C. 158oC |
| iv.Nalorphine | D. 208.5 oC |
a. i-A, ii-D, iii-C, iv-B
b. i-D, ii-A, iii-B, iv-C
c. i-C, ii-B, iii-A, iv-D
d. i-B, ii-C, iii-A, iv-D
Q.6 An example of drug from class alkylamine antihistamine drugs is?
a) Dimethindene
b) Nalorphine
c) Edrophonium
d) Phentolamine
Q.7 Type of ring present in the structure of Dimethindene is?
a) Purine
b) Indene
c) Oxazophosphorine
d) Furan
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-a
2-a
3-b
4-d
5-c
6-a
7-b