FELODIPINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
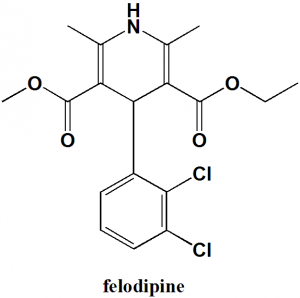
Felodipine
IUPAC nomenclature
(RS)-3-ethyl 5-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate.
Classification
- Dihydropyridines
- Calcium channel blockers
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 384.2 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | 145oC |
| 4 | Solubility | 19.7mg/L |
| 5 | Octanol/water partition coefficient | 3.86 |
| 5 | Presence of ring | Dihydropyridine, phenyl |
| 6 | Number of chiral centers | 1 |
Mechanism of Action
- The influx of calcium ions through voltage gated L-type calcium channels is inhibited by Felodipine which results in decrease in the arterial smooth muscle contractility and subsequent vasoconstriction.
- This inhibition results in overall decrease in blood pressure.
Structure Activity Relationship
General structure activity of dihydropyridines calcium channel blockers can be summarized as:
- R1 should be unsubstituted.
- R1 should be easily detachable group.
- Basic amino ethyl ether chain at R2 increases the potency of drug, while H/aryl group results in loss of activity of drug.
- Substitution of R3 and R5 with alkoxy carbonyl group gives optimum activity.
- Branching of alkyl chain of ester group decreases the activity.
- R4 must be phenyl ring.
- S-enantiomers are more active than R-enantiomers.
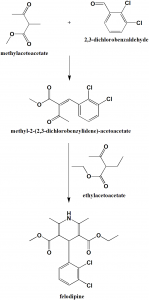
Method of synthesis
i. Methylacetoacetate reacts with 2,3-dichlorobenzaldehyde to give methyl-2-(2,3-dichlorobenzyidene)-acetoacetate.
ii. The last is reacted with ethylacetoacetate in presence of ammonia to give felodipine. [1]
Medicinal Uses
Felodipine is used for the treatment of:
- Mild hypertension
- Moderate hypertension
Side Effects
Side effects of felodipine are:
- Dizziness
- Weakness
- Flushing
- Fainting
- Lightheadedness
- Swelling of ankles
- Constipation
- Headache
- Stomach upset
- Allergic reactions
MCQs
Q.1 What can be the correct IUPAC nomenclature of Felodipine?
a) 6-chloro-1,1-dioxo-2H-1,2,4-benzothiadiazine-7-sulfonamide
b) 1,4:3,6-dianhydro-2,5-di-O-nitro-D-propanol.
c) 1-[(4-Chlorophenyl)(phenyl)methyl]-4-methylpiperazine
d) (RS)-3-ethyl 5-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
Q.2 Which amongst the following statements is/are correct related to the SAR of Dihydropyridines calcium channel blockers?
I. Branching of alkyl chain of ester group decreases the activity.
II. R4 must be phenyl ring.
III. S-enantiomers are more active than R-enantiomers.
a) I, III
b) II, III
c) I, II
d) I, II, III
Q.3 The correct order for the synthesis of drug Felodipine from Methyl acetoacetate can be?
I. Reaction with 2,3-dichlorobenzaldehyde
II. Sulfonylchlorination
III. Reaction with ammonia
IV. Reaction with ethylacetoacetate
a) I – IV
b) I – II
c) III – II
d) I – IV – III
Q.4 Side effects of drug Felodipine is/are?
a) Dizziness
b) Diarrhea
c) Muscle spasms
d) All of the above
Q.5 Match the following drugs with their correct Octanol water partition coefficient-
| i. Felodipine | A. 2.05 |
| ii. Pantaprazole | B. 1.9 |
| iii. Omeprazole | C. 3.86 |
| iv. Polythiazide | D. 2.23 |
a) i-C, ii-B, iii-A, iv-D
b) i-C, ii-A, iii-D, iv-B
c) i-A, ii-D, iii-C, iv-B
d) i-A, ii-C, iii-B, iv-D
Q.6 An example of drug from class calcium channel blockers?
a) Felodipine
b) Prazosin
c) Hydroxyamphetamine
d) Pseudoephedrine
Q.7 The type of ring system found in the structure of drug Felodipine is?
a) Dihydroopyridine
b) Benzothiazine
c) Naphthalene
d) Pyrimidine
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
ANSWERS
1-d
2-d
3-d
4-a
5-b
6-a
7-a
REFERENCES
[1] EP 311 582 (Hässle; appl. 22.9.1988; S-prior. 8.10.1987).