GABAPENTIN Synthesis, SAR, MCQ, Structure, Chemical Properties and Therapeutic Uses
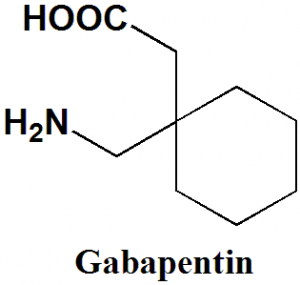
Gabapentin
IUPAC nomenclature
1-(Aminomethyl)cyclohexaneacetic acid
Classification
Gabapentin is an anticonvulsant drug.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 171.24g/mol |
| 2 | Physical appearance | White to off-white crystalline solid |
| 3 | Melting point | 162-166°C |
| 4 | Solubility | Freely soluble in water, alkaline and acidic solutions. |
| 5 | Octanol/water partition coefficient | 1.25 |
| 6 | Presence of ring | Cyclohexyl ring |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
Gabapentin inhibits the action of α2δ-1 subunits. Due to this, there is decrease in density of pre-synaptic voltage-gated calcium channels and subsequent release of excitory neurotransmitters. This inhibition is also responsible for the anti-epileptic action of the drug.
Structure Activity Relationship
- Substitution of the isopropyl group with diethyl decreases affinity for α2δ by more than 15 fold.
- 1-ethylpropyl analogues are 2.5 fold weaker at α2δ but having the same potency at inhibiting system L transport of leucine.
- Transposition of the isobutyl side chain to the C-2 position reduces affinity for α2δ and system L transporter. Changes also led to loss of in vivo activity in the epilepsy, pain and anxiety models.
- Substitution of a methyl group α to the carboxylic acid leads to complete loss of affinity for α2δ subunit. There is little effect on system L transporter affinity.
- Substitution with methyl or ethyl groups at C-3 position causes 40 to 100-fold decrease in α2δ affinity and 3 to 5-fold increase in affinity for system L transporter.
- Introduction of a methyl group α to the amine group and trans to the 3-isobutyl group enhances the α2δ affinity by 2-fold and decrease in system L transport potency.
- Original isobutyl side chain is optimal side chain at the C-3 position.
- Introducing the methyl group α to the amine in a syn orientation increases potency of α2δ by 2-folds while, there is complete loss of activity at the system L amino acid transporter. [1]
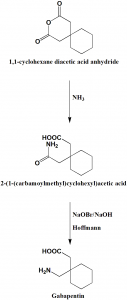
Method of synthesis
i. 1,1-cyclohexane diacetic acid anhydride is converted to 1,1-cyclohexane diacetic acid monoamide by reacting the former with a composition that produces free ammonia in solution
ii. 1,1-cyclohexane diacetic acid monoamide undergoes Hoffmann reaction to produce gabapentin. [2]
Therapeutic Uses
Gabapentin is used for:
- Prevention and control of seizures
- Relieving nerve pain
Side Effects
Side effects of Gabapentin are:
- Dizziness
- Drowsiness
- Blurred vision
- Double vision
- Tremors
- Depression
- Suicidal thoughts
- Mood changes
MCQs
Q.1 Choose the correct statements related with the physicochemical properties of drug Gabapentin.
I. Molecular weight = 278.19 gm/mol
II. It is white to off-white crystalline solid
III. Melting point is 215oC
IV. It is freely soluble in water
a) I, III, IV
b) II, IV
c) I, IV
d) I, II, IV
Q.2 Match the following of the drugs with their correct IUPAC names.
| i. Gabapentin | A. 8-chloro-6-(4-methylpiperazin-1-yl)benzo[b][1,4]benzoxazepine |
| ii. Loxapine | B. 5H-dibenzo[b,f]azepine-5-carboxamide |
| iii. Carbamazepine | C. 1-(Aminomethyl)cyclohexaneacetic acid |
| iv. Thiopental | D. 5-ethyl-5-pentan-2-yl-2-sulfanylidene-1,3-diazinane-4,6-dione |
a) i-C, ii-A, iii-B, iv-D
b) i-D, ii-A, iii-C, iv-B
c) i-D, ii-A, iii-B, iv-C
d) i-A, ii-C, iii-B, iv-D
Q.3 Correct steps for the mechanism of action of the drug Gabapentin are?
I. Inhibition of the action of α2δ-1 subunits
II. Stimulation of the action of α2δ-1 subunits
III. Increase in density of pre-synaptic voltage-gated calcium channels
IV. Decrease in density of pre-synaptic voltage –gated calcium channels
a) I – IV
b)II – III
c) III – I
d) IV- II
Q.4 Correct sequence for True/false for the classification of the drug can be?
- Gabapentin: Anticonvulsant drug
- Metocurine: Muscarnic agonist
- Cevimeline: Acetylcholine antagonist
- Succinylcholine: Nicotinic antagonist
a) TFFT
b) TTTT
c) TFFT
d) FFTF
Q.5 Original isobutyl side chain of the gabapentin is?
a) Optimal at C-2 position
b) Optimal at C-3 position
c) Produces optimal activity at C-5 position
d) Produces optimal activity at C-4 position
Q.6 Correct steps for the synthesis of gabapentin from 1,1-cyclohexane diacetic acid anhydride are?
I. Reduction
II. Hoffman reaction
III. Reaction with free ammonia
IV. Treatment with Baeyer’s reagent
a) II, IV
b) II, IV
c) I, III
d) III, II
Q.7 Side effect of drug Gabapentin include?
a) Hyperactivity
b) Shallow breathing
c) Increase in blood counts
d) Tremors
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-b
2-a
3-a
4-a
5-b
6-d
7-d
REFERENCES
[1] ] Belliotti TR, Capiris T, Ekhato IV, Kinsora JJ, Field MJ, Heffner TG, Meltzer LT, Schwarz JB, Taylor CP, Thorpe AJ, Vartanian MG. Structure− activity relationships of Pregabalin and analogues that target the α2-δ protein. Journal of medicinal chemistry. 2005 Apr 7;48(7):2294-307. [2] Kumar A, Soudagar SR, Nijasure AM, Panda NB, Gautam P, Thakur GR, inventors; Ipca Laboratories, assignee. Process For Synthesis Of Gabapentin. United States patent application US 11/923,352. 2008 May 1.