IMATINIB Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
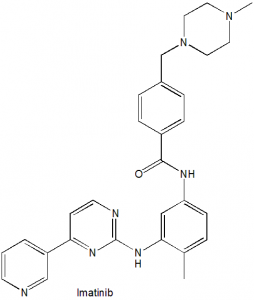
Imatinib
IUPAC nomenclature
4-[(4-methylpiperazin-1-yl)methyl]-N-(4-methyl-3-{[4-(pyridin-3-yl)pyrimidin-2-yl]amino}phenyl)benzamide.
Classification
Imatinib falls under the category of antibiotic Antineoplastic cytotoxic drug. [1]
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 493.6 g/mol |
| 2 | Appearance | Present in solid form |
| 3 | Melting point | 226°C |
| 4 | Solubility | Very soluble in water |
| 5 | Octanol/water partition coefficient | 3 |
| 6 | Presence of ring | Imatinib consists of a pyrimidine ring, aminopyrimidine ring, methylbenzene ring, benzamide ring and N-methlpiperazine ring. |
Mechanism of Action
i. It inhibits the Bcr-Abl tyrosine kinase.
ii. This inhibits the proliferation and thus, induction of apoptosis in cell.2]
Structural Activity Relationship
- Amide group as a substituent at phenyl group shows inhibitory action against tyrosine kinases.
- Substitution at 6 position of diaminophenyl ring abolished the activity against PKC.
- Introduction of methyl group in an ortho position to the amino group can increase the selectivity of Bcr-Abl.
- Bioavailablity and solubility can be enhanced by the introduction of N-methylpiperazine group. [2]
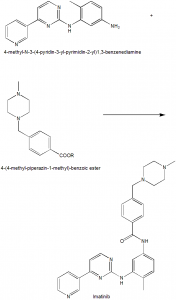
Method of synthesis
Imatinib can be synthesized by the reaction of 4-methyl-N-3-(4-pyridine-3-yl-pyrimidine-2-yl)-1,2-benzenediamine with 4-(4-methyl-piperazine-1-methyl)-benzoic ester in the presence of base in a non-protonic organic solvent. [3]
Therapeutic Uses
Imatinib is given for the treatment of:
- Philadelphia chromosome positive chronic myeloid leukemia.
- Philadelphia chromosome positive acute lymphoblastic leukemia
- Myelodysplastic/ myeloproliferative diseases associated with PDGFR gene rearrangements.
- Gastrointestinal stromal tumors which are C-kit positive.
Side Effects
- Common side effects of imatinib include fever, low blood count, skin rashes, hemorrhage, diarrhea, muscle cramps, bone pain, Edema, Nausea and vomiting.
- Less common side effects are headache, infertility, weakness, nose bleeds, night sweats, constipation, poor appetite, shortness of breath, coughing, abdominal pain, indigestion and joint pain.
MCQs
Q.1 The term ‘Gleevek’ is associated with which drug?
a) Triptorelin
b) Imatinib
c) 5-FU
d) 6-TG
Q.2 Introduction of methyl group in an ortho position to the amino group can ………. the selectivity of Bcr-Abl.
a) Increase
b) Decrease
c) Either increase or decrease
d) Do not change selectivity in either ways
Q.3 Which of the following are the correct side effects of the drug Imatinib?
I. High blood counts
II. Infertility
III. Shortness of breath
IV. Indigestion
a) I, II & IV
b) I, II, III & IV
c) II, III & IV
d) I, III & IV
Q.4 The starting chemicals required for the synthesis of drug Imatinib are?
a) 4-methyl-N-3-(3-yl-pyrimidine-2-yl)-1,2-benzenediamine and 4-(4-methyl-piperazine-1-methyl)-benzoic ester
b) 4-methyl-N-3-(4-pyridine-3-yl-)-1,2-benzenediamine with 4-(4-methyl-piperazine-1-methyl)-benzoic ester
c) 4-methyl-N-3-(4-pyridine-3-yl-pyrimidine-2-yl)-1,2-benzenediamine with 4-(4-methyl-piperazine-1-methyl)-butanoic ester
d) 4-methyl-N-3-(4-pyridine-3-yl-pyrimidine-2-yl)-1,2-benzenediamine with 4-(4-methyl-piperazine-1-methyl)-benzoic ester
Q.5 Which pairs of drug and its classification are true?
| I. | Topotecan | Captothecin analogues |
| II. | Vincrisine | Progestins |
| III. | Imatinib | Antibiotic |
| IV. | Carmustine | Glucocorticoids |
a) I
b) II & III
c) I & III
d) I & IV
Q.6 Correct physical form in which the drug Imatinib is found at normal temperature and pressure?
a) Solid form
b) Solid crystalline form
c) Liquid form
d) Gaseous form
Q.7 Which of the following rings is NOT present in the structure of Imatinib?
a) Pyrimidine ring
b) Pteridine ring
c) Benzamide ring
d) Aminopyrimidine ring
ANSWERS
1-b
2-a
3-c
4-d
5-a
6-a
7-b
REFERENCES
[1] Tripathi KD. Essentials of Medical Pharmacology, 6thEdn. Jaypee Brothers Medical Publishers (P) Ltd. 2008: 820. [2] Rossari F, Minutolo F, Orciuolo E. Past, present, and future of Bcr-Abl inhibitors: from chemical development to clinical efficacy. Journal of hematology & oncology. 2018 Dec;11(1):84. [3] Kankan RN, Rao DR, inventors; Cipla Ltd, assignee. Process of preparing imatinib and imatinib prepared thereby. United States patent US 7,638,627. 2009 Dec 29.