LANSOPRAZOLE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
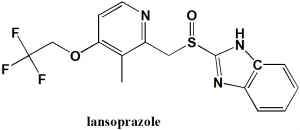
Lansoprazole
IUPAC nomenclature
2-[[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methylsulfinyl]-1H-benzimidazole
Classification
- Proton pump inhibitors
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 369.4 g/mol |
| 2 | Physical appearance | White to brownish-white crystalline powder |
| 3 | Melting point | 178-182oC |
| 4 | Solubility | Freely soluble in dimethylformamide; insoluble in hexane and water |
| 5 | Octanol/water partition coefficient | N/A |
| 5 | Presence of ring | Pyridine. Benzimidazole |
| 6 | Number of chiral centers | Not present |
Mechanism of Action
- Lansoprazole is a prodrug which requires protonation via an acidic environment to get activated.
- Lansoprazol covalently binds with cystien residues via disulfide bridges on the α subunit of H+/K+ ATPase enzyme system.
- Thereby it inhibits the H+/K+ ATPase pump which in turn inhibits the gastric acid secretion or formation of hydrochloric acid in parietal cells
Structure Activity Relationship
General structure activity of Heteroaryl- and heterocyclyl-substituted imidazo[1,2-a]Pyridine derivatives acting as acid pump antagonists can be summarized as:
- The inhibitory property of drugs depends on hydrophobic group substituted at the left side ortho-position of the phenyl ring.
- Increase in hydrophobic value increases the activity.
- Increasing the GTCI value decreases the activity.
- Small molecule substitutions may participate in hydrophobic interaction as well as steric interactions. [1]
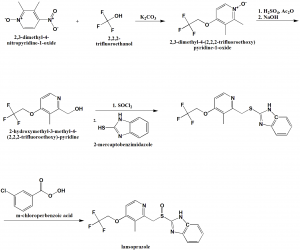
Method of synthesis
i. 2,3-dimethyl-4-nitropyridine-1-oxide is reacted with 2,2,2-trifluoroethanol in presence of potassium carbonate to give 2,3-dimethyl-4-(2,2,2-trifluoro-ethoxy)pyridine-1-oxide.
ii. The compound so formed is treated with acetic anhydride in acidic conditions followed by nutrilizing with sodium hydroxide solution to get 2-hydroxymethyl-3-methyl-4-(2,2,2-trifluoroethoxy)-pyridine.
iii. Last is treated with thionyl chloride followed by reaction with 2-mercaptobenzimidazole to get 2-[3-methyl-4-(2,2,2-trifluoroethoxy)pyrid-2-ylmethylthio]benzimidazole.
iv. Above formed compound is reacted with m-chloro-perbenzoic acid to get lansoprazole.[2]
Medicinal Uses
Lansoprazole is used for treatment of:
- Stomach ulcers
- Active duodenal ulcers
- To reduce the risk of duodenal ulcers by eradicating Helicobacter
- Active benign gastric ulcer
- GERD
- Erosive esophagitis
- Zollinger-Errison syndrome
- Reduce heart burn symptoms
- NSAID induced gastric ulcers
Side Effects
Side effects of Lansoprazole are:
- Abdominal pain
- Nausea
- Constipation
- Headache
- Low magnesium blood level
- Irregular heartbeat
- Muscle spasms
- Seizures
- Lupus
- Diarrhea
- Blood in stool
MCQs
Q.1 Correct statements from the following related with the physicochemical properties of drug Lansoprazole are?
I. Molecular weight: 474.9 gm/mol
II. Appearance: Oil
III. Melting Point: 190 oC
IV. Solubility: Isoluble in water
a) I, II, III
b) I, III, IV
c) IV
d) I, II, III, IV
Q.2 Match the following of the drugs with their correct IUPAC names.
| i. Lansoprazole | A. (RS)-6-(Difluoromethoxy)-2-[(3,4-dimethoxypyridin-2-yl)methylsulfinyl]-1H-benzo[d]imidazole |
| ii. Isoxicam | B. 4-hydroxy-2-methyl-N-(5-methyl-1,2-oxazol-3-yl)-1,1-dioxo-1λ6,2-benzothiazine-3-carboxamide |
| iii. Doxylamine | C. (RS)-N,N-dimethyl-2-(1-phenyl-1-pyridin-2-yl-ethoxy)-ethanamine |
| iv. Pentoprazole | D. 2-[[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methylsulfinyl]-1H-benzimidazole |
a) i-D, ii-B, iii-C, iv-A
b) i-A, ii-B, iii-D, iv-C
c) i-B, ii-C, iii-A, iv-D
d) i-A, ii-C, iii-D, iv-B
Q.3 Mechanism of action of drug Lansoprazole involves?
I. Protonoation to get activated
II. Inverse agonist for H1 receptor
III. Inhibition of the H+/K+ ATPase pump
a) I, III
b) I, IV
c) I, II, III
d) II, III
Q.4 Correct sequence for True/false for the classification of the drug can be?
- Lansoprazole: H1-receptor antihistamine drug
- Chlorprothixene: Sedative-hypnotics
- Omeprazol: H1-antagonist
- Desflurane: Corticoids
a) TFTF
b) TTFF
c) TFTT
d) FFFF
Q.5 In Heteroaryl- and heterocyclyl-substituted imidazo[1,2-a]Pyridine derivatives acid-pump antagonists, inhibitory property depends on?
a) Hydrophobic group position
b) GTCIvalue
c) Hydrophobic value
d) All of the above
Q.6 Type of rings present in the structure of Lansoprazole?
I. Pyridine
II. Piperazine
III. Benimidazole
IV. Benzodiazepine
a) I, IV
b) I, II
c) I, III
d) II
Q.7 Side effect of drug Lansoprazole is/are?
a) Nausea
b) Seizures
c) Diarrhea
d) All of the above
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
ANSWERS
1-c
2-a
3-a
4-d
5-d
6-c
7-d