Mechanism of Enzyme Action and Specificity of Enzymes and MCQs
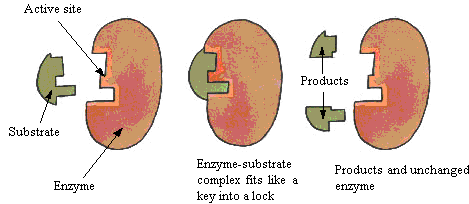
Formation of enzyme substrate complex is considered as the first step of enzyme catalysis. Substrate binds to the active site of the enzyme through various non-covalent interaction and thus forms ES complex. The ES complex then further is converted to the product and free enzyme.
The active site of the enzyme is the region where the substrate binds. The substrate contains specific amino acid residues:
- Binding residue: It recognizes and binds the substrate to form ES complex
- Catalytic residue: Creates the chemical environment that enhances the rate reactions and further converts the ES complex into product.
Any change in the primary, secondary, tertiary and quaternary structure can affect the active site and thus reduces the catalytic activity.
The two models that propose the binding of substrate on the active site of the enzyme are:
Lock and key model or rigid template model of Emil Fisher
This model was prepared in 1890 by Emil Fisher. In this model, the enzyme is pre-shaped and the active site has rigid structure which is complementary to that of the substrate. This is called lock and key model because the substrate fits on the active site of the enzyme in the same way as the key fits in the lock.
This model describes that how enzyme binds only to particular specific enzyme and will not bind to any other substrate with almost identical structure. This model explains all the mechanism but do not explain the changes in enzyme activity in the presence of allosteric modulators.
Induced fit model or hand-in glove model of Daniel E koshland
This model explains both the aspects:
- Specificity of enzyme substrate interactions
- Dynamic changes that takes place during catalysis
This model was proposed in 1958 which stated that enzymes are flexible and the shape of the active site can be modified by the binding of the substrate.

Specificity of enzyme action
Specificity of the enzyme allow it bind with the appropriate substrate. Enzymes are specific in both catalytic reaction as well as the substrates. Specificity makes it possible for the number of enzymes to co-exist in a cell without interfering each other’s functions.
Types of specificity
There are three types of specificity:
- Substrate specificity: these are further divided into three
- Absolute substrate specificity: these act only on one substrate and catalyzes only one reaction
- Relative substrate specificity: in this case, enzyme act on more than one substrate
- Broad substrate specificity: these enzyme act on more than one structurally related substrate
- Reaction specificity:- in this case, enzyme is specific to particular reaction but not the substrate and catalyzes only one type of reaction
- Stereo specificity:- many enzymes are specific towards the stereoisomers that is they act only on one type of isomer.
Multiple Choice Questions (MCQs)
1. How many models are there which describes are mechanism of enzyme action?
A. 2
B. 3
C. 4
D. 5
2. What is the active site of the enzyme?
A. Where modulators bind
B Where substrate bind
C. Both
D. None of the above
3. When was lock and key model proposed?
A. 1985
B. 1850
C. 1890
D. 1958
4. When was hand-in glove model proposed?
A. 1985
B. 1850
C. 1890
D. 1958
5. Change in which type of enzyme structure reduces the catalytic activity?
A. Primary
B. Secondary
C. Tertiary
D. All of the above
6. In Which of the following model, enzyme is considered as pre-shaped?
A. Lock and key
B. Induced fit model
C. Both
D. None of the above
7. After the formation of which complex, product is formed?
A. EI
B. ES
C. EP
D. None of the above
8. Match the following-
a. Absolute substrate specificity 1. Enzyme specific towards stereo isomers
b. Broad substrate specificity 2. Enzyme specific towards reactions
c. Reaction specificity 3. Enzyme act on more than one structurally similar enzyme
d. Stereo specificity 4. Act only on one substrate
9. In Which of the following model, enzyme is considered as flexible?
A. Lock and key
B. Induced fit model
C. Both
D. None of the above
10. Who proposed rigid template model?
A. James Watson
B. Emil Fisher
C. Daniel E Koshland
D. Daniel Fisher
11. Specificity of enzyme is divided in how many types?
A. 2
B. 3
C. 4
D. 5
12. Which of the following statement is NOT true?
A. Changes in quaternary structure reduces the catalytic activity
B. Substrate binds on the allosteric site
C. Specificity allows the enzymes to co-exist in the same cell without any interference
D. Lock and key model is also known as rigid template model
13. The substrates contain how many amino acid residues?
A. 2
B. 3
C. 4
D. 5
14. Who proposed induced fit model?
A. James Watson
B. Emil Fisher
C. Daniel E Koshland
D. Daniel Fisher
15. Which model explains the dynamic changes during the catalysis?
A. Lock and key
B. Rigid template model
C. Both
D. None of the above
ANSWERS:-
1. 2
2. Where substrate bind
3. 1890
4. 1958
5. All of the above
6. Lock and key
7. ES
8. a – 4 b – 3 c – 2 d – 1
9. Induced fit
10. Emil Fisher
11. 3
12. Substrate binds on the allosteric site
13. 2
14. Daniel E Koshland
15. None of the above