NABUMETONE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Nabumetone
IUPAC nomenclature
4-(6-methoxy-2-naphthyl)-2-butanone
Classification
- NSAID
- Nonacidic prodrug
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 228.29 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | 78-82 °C |
| 4 | Solubility | Practically insoluble |
| 5 | Octanol/water partition coefficient | 3.1 |
| 6 | Presence of ring | Naphthalene |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
- Nabumetone converts into its active metabolite 6-MNA which inhibits COX-1 and COX-2 with greater selectivity for COX-2 enzyme.
- This results in reduction of the conversion of the arachidonic acid to prostaglandins and thromboxane.
Structure Activity Relationship
SAR of class Pyranocarboxylic acid can be summarized as follows:
- Introduction of methyl or ethylgroups on the butanone side chain decreases the anti-inflammatory activity of drug.
- Conversion of ketone group into dioxolane retains the activity.
- Conversion of ketone into oxime group decreases the activity
- Activity is reduced on removing the methoxy group from the 6th-position.
- Replacement of the methoxy group at 6th position with methyl or chloro group retains the activity.
- Replacement of methoxy group at 6th-position with acetoxy, hydroxyl, N-methylcarbamoyl groups decreases the activity.[1]
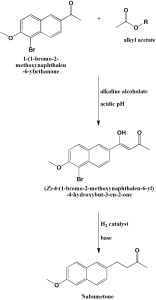
Method of synthesis
i. 2-acetyl-5-bromo-6-methoxynaphthalene is reacted with alkyl acetate in presence of alkaline alcoholate to get 4-(5-bromo-6-methoxy-2-naphthyl)-4-hydroxybut-3-en-2-one.
ii. Last undergoes catalytic hydrogenation with hydrogen at 1-10 atm pressure.
iii. The product is filtered and concentrated under vacuum condition to get the oily substance.
iv. The oily substance undergoes crystallization by alcohols and forms bisulfitic complex with sodium bisulfate in polar solvents.
v. The bisulfitic complex is treated with aqueous solution of hydroxide, carbonate or bicarbonate of an alkali metal to restore nabumetone.[2]
Therapeutic Uses
Nabumetone is used for:
- Treatment of osteoarthritis
- Treatment of rheumatoid arthritis
Side Effects
Side effects of nabumetone are:
- Allergic reactions
- Skin reactions
- Increasing the risk of fatal heart attack or stroke
- Chest pain
- Numbness
- Weakness
- Shortness of breath
- Swelling of legs
- Slurred speech
- Vision changes
- Rapid weight gain
- Stomach bleeding
- Nausea
- Loss of appetite
- Jaundice
- Pale skin
- No urination
- Anemia
- Nausea
- Vomiting
- Indigestion
- Diarrhea
- Constipation
- Dizziness
- Headache
- Tiredness
- Ringing sounds in ears
MCQs
Q.1 Mechanism of action of nabumetone is due to?
a) Inhibition of COX enzyme
b) Inhibition of Acetylcholinestrase
c) Alkylation of the genetic material
d) Stimulating α-adrenoceptors
Q.2 Therapeutic use of drug nabumetone is/are?
a) Treatment of oesteoarthritis
b) Treatment of malignant tumors
c) As an anesthetic agent
d) All of the above
Q.3 Which amongst the following are the correct statements with respect to the SAR of class Pyranocarboxylic acids?
I. Introduction of methyl or ethylgroups on the butanone side chain decreases the anti-inflammatory activity of drug.
II. Conversion of ketone group into dioxolane retains the activity.
III. Conversion of ketone into oxime group decreases the activity
IV. Activity is reduced on removing the methoxy group from the 6th-position.
a) I, II, III, IV
b) II, IV
c) I, III, IV
d) II, III
Q.4 Number of chiral carbons present in the structure of nabumetone is?
a) 0
b) 1
c) 2
d) 3
Q.5 Correct sequence for the True/False for the physiochemical properties of the drug Nabumetone?
- Molecular weight:228.29 gm/mol
- Physical appearance: Solid
- Melting point: 78-820C
- Octanol/water partition coefficient: 3.1
a) TFFT
b) FFTF
c) TTTT
d) FFFF
Q.6 Correct statements for the IUPAC nomenclatures of the drugs are?
I. Nabumetone: 4-(6-methoxy-2-naphthyl)-2-butanone
II. Cisplatin: (SP-4-2)-diamminedichloroplatinum(II)
III. Mitoxantron: 1,4-dihydroxy-5,8-bis[2-(2-hydroxyethylamino)ethylamino]-anthracene-9,10-dione.
IV. 6-Thioguanine: 2-amino-1H-purine-6(7H)-thione
a) I, III
b) II, III, IV
c) I, IV
d) I, II, III, IV
Q.7 Match the following drugs with their correct classifications-
| i. Nabumetone | A. Muscarinic antagonist |
| ii. Darifenacin | B. NSAID |
| iii. Bisoprolol | C. Cholinergic agonist |
| iv. Acetylcholine | D. ß-adrenergic antagonist |
a) i-A, ii-D, iii-B, iv-C
b) i-B, ii-A, iii-D, iv-C
c) i-B, ii-C, iii-A, iv-D
d) i-A, ii-B, iii-C, iv-D
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-a
2-a
3-a
4-a
5-c
6-d
7-b