NAPROXEN Synthesis, SAR, MCQ, Structure, Chemical Properties and Therapeutic Uses
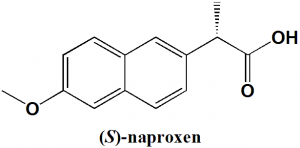
Naproxen
IUPAC nomenclature
(+)-(S)-2-(6-Methoxynaphthalen-2-yl)propanoic acid
Classification
- NSAID
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 230.6 g/mol |
| 2 | Physical appearance | White to off-white crystalline powder |
| 3 | Melting point | 153°C |
| 4 | Solubility | Soluble in methanol, chloroform; slightly soluble in ether |
| 5 | Octanol/water partition coefficient | 3.18 |
| 6 | Presence of ring | Naphthalene |
| 7 | Number of chiral centers | 1 |
Mechanism of Action
- Naproxen inhibits COX-2 enzyme which results in decrease in production of prostaglandins which is responsible for conveying of pain and inflammation signals in the body.
- It also inhibits COX-1 enzyme which is responsible for the side effects of drug like GI ulcerations.
Structure Activity Relationship
SAR of naproxen can be summarized as follows:
- Maximum activity was found at substitution in the 6th – position.
- Small lipophillic groups are active analogues with CH3O is most potent.
- Derivatives with larger group substitutions are found to be les active.
- 2-naphthylpropionic acid derivatives were found to be more active than corresponding acetic acid analogues.
- Replacing the carboxyl group with such groups that are capable of getting metabolized to the carboxyl groups retains the activity.
- (+)-S-enantiomer is found to be more potent.[1]
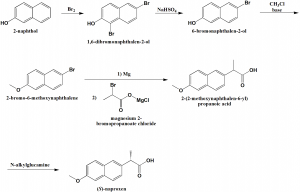
Method of synthesis
i. 2-naphthol undergoes bromination to give 1,6-dibromonaphthalen-2-ol.
ii. The above formed compound is reacted with sodium bisulfate to give 6-bromonaphthalen-2-ol.
iii. The last is alkylated using methylchloride in presence of base to give 2-bromo-6-methoxynaphthalene.
iv. On reaction with magnesium followed by reaction with magnesium 2-bromopropanoate chloride, naproxen is produced.
v. (S)-enantiomer of naproxen is separated by N-alkylglucamine. [2]
Therapeutic Uses
Naproxen is used for:
- Relieving mild to severe pain and inflammation due to arthritis, bursitis, gout, menstrual cramps and tendinitis.
Side Effects
Side effects of Naproxen are:
- Allergic reactions
- Skin reactions
- Shortness of breath
- Heartburn
- Weight gain
- Vision changes
- Indigestion
- Pale skin
- No urination
- Anemia
- Nausea
- Vomiting
- Dizziness
- Headache
- Tiredness
- Ringing sounds in ears
MCQs
Q.1 Which statements are correct with respect to the physicochemical properties of drug Naproxen?
I. Molecular weight:408.09gm/mol
II. Appearance: White to off-white crystalline powder
III. Melting point: 153oC
a) I, II
b) II, III
c) I, II, III
d) I, III
Q.2 Match the following of the drugs with their correct IUPAC names.
| i. Naproxen | A. 5,5-diethylpyrimidine-2,4,6(1H,3H,5H)-trione |
| ii. Methsuximide | B. (RS)-1,3-dimethyl-3-phenyl-pyrrolidine-2,5-dione |
| iii. Barbital | C. (RS)-N’-(7-chloroquinolin-4-yl)-N,N-diethyl-pentane-1,4-diamine
|
| iv. Chloraquine | D. (+)-(S)-2-(6-Methoxynaphthalen-2-yl)propanoic acid |
a) i-C, ii-D, iii-A, iv-B
b) i-D, ii-B, iii-A, iv-C
c) i-C, ii-A, iii-D, iv-B
d) i-B, ii-C, iii-D, iv-A
Q.3 Mechanism of action of Naproxen includes?
I. Inhibition of COX-1 enzyme
II. Inhibition of COX-2 enzyme
III. Decrease in production of prostaglandins
IV. Reduces pain and inflammation signal transmission
a) II, IV
b) I, II, III, IV
c) I, III
d) II, III, IV
Q.4 Correct sequence for True/false for the classification of the drug can be?
- Naproxen: NSAID
- Dactinomycin: Antibiotics
- BCNU: Nitrosoareas alkylating agent
- Methohexital: Anesthetics
a) TFFT
b) FTFT
c) TTTT
d) TTFF
Q.5 An incorrect statement related with the SAR of naproxen is?
a) Maximum activity was found at substitution in the 6th – position.
b) Small lipophillic groups are active analogues with CH3O is most potent.
c) Derivatives with larger group substitutions are found to be more active.
d) 2-naphthylpropionic acid derivatives were found to be more active than corresponding acetic acid analogues.
Q.6 Type of ring present in the structure of Naproxen?
I. Anthracene
II. Naphthaline
III. Phenyl
IV. Furan
a) I, III
b) II
c) I, III, IV
d) No ring structure present
Q.7 Side effect of drug Naproxen?
a) Vision changes
b) Ingestion
c) Anemia
d) All of the above
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-c
2-b
3-b
4-c
5-c
6-b
7-d