NITRAZEPAM Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
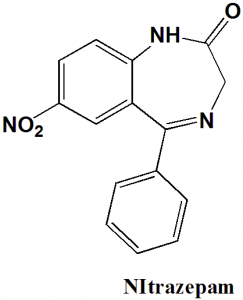
Nitrazepam
IUPAC nomenclature
7-nitro-5-phenyl-1H-benzo[e][1,4]diazepin-2(3H)-one.
Classification
Nitrazepam is a benzodiazepine sedative-hypnotic.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 281.27 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | 225°C |
| 4 | Octanol/water partition coefficient | 2.25 |
| 5 | Solubility | >42.2 [ug/mL] |
| 6 | Presence of ring | Diazepine, benzene |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
i. Nitrazepam binds nonspecifically with benzodiazepine receptors BNZ1.
ii. It coupled with GABAA receptors and increases the GABA affinity for the GABA receptor.
iii. This results in opening of the chloride channel and thus, causes hyperpolarization of the cell membrane which prevents further excitation of the cell.
Structure Activity Relationship
- Ring A should include an aromatic or heteroaromatic ring for binding with 5-phenyl-1,4-benzodiazepin-2-one derivatives.
- An electronegative group at 7-position of the ring A increases the functional anxiolytic activity.
- Substitutions at 6, 8 or 9 position with electronegative group on ring A will decrease the functional anxiolytic activity.
- When Heterocycles used as ring A, drug shows poor pharmacological activity.
- A proton-accepting group is essential on Ring B for binding with GABAA
- When the proton accepting group is present on the 2-position of the ring B, and is in coplanar spatial orientation with Ring A, maximum activity is observed.
- Replacement of oxygen with sulfur in ring B results in alteration in the selectivity for binding with GABA BZR subpopulations, but anxiolytic properties are maintained.
- There is no effect on agonist activity, but the antagonist activity decreases when methylene 3-position or imine nitrogen of the ring B is substituted.
- Derivatives having the 3-hydroxy moiety are fast excreted.
- Sterically large substituents on ring B, like tert-butyl group reduces the receptor affinity and the in vivo activity.
- 4,5-double bond and 4-position nitrogen is not essential for anxiolystic activity.
- BZR affinity is decreased if C=N bond is replaced with C-N bond.
- 5-phenyl ring C is not necessary for the binding with BZR.
- Substitution at the para position of the ring C decreases the agonist activity of the drug.
- There is no change observed in the agonist property of the drug when there is substitution at ortho position.
- When 1,2-bond f the ring C is annelated with an additional electron rich ring such as imidazole, affinity of the BZR increases. [1]
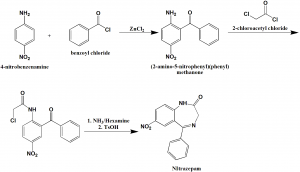
Method of synthesis
i. Condensation of nitroaniline, benzoyl chloride and zinc chloride gives (2-amino-5-nitrophenyl)(phenyl)methanone.
ii. On reaction with 2-chloroacetyl chloride, a compound is formed which on reaction with ammonia followed by reaction with tosyl hydroxide produces Nitrazepam.
Therapeutic Uses
Nitrazepam is used for:
- Treatment of insomnia
Side Effects
Side effects of Nitrazepam are:
- Dizziness
- Memory loss
- Hallucinations
- Confusion
- Mood changes
- Anxiety
- Aggressive behavior
- Loss of coordination
MCQ
Q.1 ‘7-nitro-5-phenyl-1H-benzo[e][1,4]diazepin-2(3H)-one’ is the IUPAC nomenclature of which drug?
a) Nitrazepam
b) Diazepam
c) Clobazam
d) Mephobarbital
Q.2 Molecular weight of Nitrazepam?
a) 281.27 gm/mol
b) 350.74 gm/mol
c) 590.78 gm/mol
d) 208.33 gm/mol
Q.3 Match the following with correct classifications of the drugs.
| i. Nitrazepam | A. Benzodiazepine |
| ii. Asenapine | B. Butyrophenone class |
| iii. Zaleplon | C. Benzapene |
| iv. Droperidol | D. Nonbenzodiazepine agonist |
a) i-A, ii-C, iii-D, iv-B
b) i-C, ii-A, iii-D, iv-B
c) i-D, ii-A, iii-B, iv-C
d) i-B, ii-D, iii-A, iv-C
Q.4 Correct steps for the mechanism of action of the drug Nitrazepam?
I. Binding with GABA receptors
II. Hyperpolarization and stabilization of membrane
III. Increase in duration of chloride ionopore opening
a) I – III – II
b) I – II – III
c) III – II – I
d) II – I – III
Q.5 Correct sequence for True and False for the given statements related with the SAR of drug Nitrazepam?
- An electronegative group at 7-position of the ring A Increases the functional anxiolytic activity.
- Substitutions at 6, 8 or 9 position with electronegative group on ring A will Decreases the functional anxiolytic activity.
- When Heterocycles used as ring A, drug shows poor pharmacological activity.
- A proton-accepting group is not essential on Ring B for binding with GABAA
a) TFTF
b) FFTT
c)TTTF
d) FFTF
Q.6 Number of chiral centers present in Nitrazepam is?
a) 1
b) 2
c) 3
d) 0
Q.7 The drug Nitrazepam is mainly used for?
a) Treatment of hallucinations
b) Treatment of Insomnia
c) Treatment of Parkinson’s disease
d) All of the above
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-a
2-a
3-a
4-a
5-c
6-d
7-b
REFERENCES
[1] Lemke TL, Zito SW, Roche VF, Williams DA. Essentials of Foye’s principles of medicinal chemistry. Wolters Kluwer; 2017, 473-474.