OXATOMIDE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
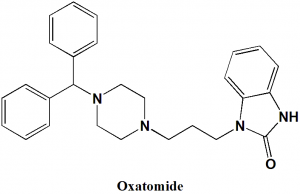
Oxatomide
IUPAC nomenclature
1-{3-[4-(diphenylmethyl)piperazin-1-yl]propyl}-1,3-dihydro-2H-benzimidazol-2-one
Classification
- H1-receptor antihistamine
- Piperazine antihistamine
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 426.6 g/mol |
| 2 | Physical appearance | N/A |
| 3 | Melting point | 153.6oC |
| 4 | Solubility | N/A |
| 5 | Octanol/water partition coefficient | N/A |
| 5 | Presence of ring | Piperazine, imidazolone, phenyl |
| 6 | Number of chiral centers | Not present |
Mechanism of Action
- Oxatomide produces antagonistic effects at H1 receptors.
- It also has antiserotonergic activity.
Structure Activity Relationship
Structure activity of piperizine antihistamines can be summarized as:
- These are the derivatives of ethylene diamines.
- The connecting moiety is CHN group
- Primary structural difference is nature of para aromatic ring substituent
- These are moderately potent.
- Slow onset of action
- Low incidence of drowsiness
- They also exhibit peripheral and central antimuscarinic activity.
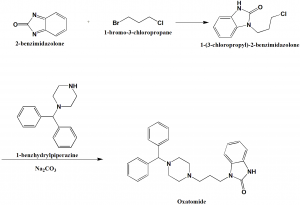
Method of synthesis
i. 2-benzimidazolone is reacted with 1-bromo-3-chloropropane to give 1-(3-chloropropyl)-2-benzimidazolone.
ii. The last is reacted with 1-benzhydrylpiperazine in presence of sodium carbonate to give oxatomide. [1]
Medicinal Uses
Oxatomide is used for treatment of:
- Allergic rhinitis
- Chronic urticaria
Side Effects
Side effects of Oxatomide are:
- Drowsiness
- Vomiting
- Dry mouth
- Fatigue
- Headache
- Difficulty in breathing
- Swelling of face
MCQs
Q.1 Match the following with correct SAR of the piperizine antihistamine drugs:
| i. They are derivatives of | A. Ethylene diamine |
| ii. Connecting moiety in drug is | B. Pyrimidines |
| C. CHO | |
| D. CHN |
a) i-A, ii-C
b) i-A, ii-D
c) i-B, ii-C
d) i-B, ii-D
Q.2 Correct sequence for the True/False for correct IUPAC names of the drug can be?
- Oxatomide: 1-{3-[4-(diphenylmethyl)piperazin-1-yl]propyl}-1,3-dihydro-2H-benzimidazol-2-one
- Tetracaine: 2-(diphenylmethoxy)-N,N-dimethylethanamine
- Clemastine: (2R)-2-{2-[(1R)-1-(4-chlorophenyl)-1-phenylethoxy]ethyl}-1-methylpyrrolidine
- Methimazole: 4-hydroxy-2-methyl-N-(5-methyl-1,3-thiazol-2-yl)-1,1-dioxo-1λ6,2-benzothiazine-3-carboxamide
a) TFFT
b)TFTF
c) TTTT
d) FFFT
Q.3 Molecular weight of Oxatomide is?
a) 426.6 gm/mol
b) 140.6 gm/mol
c) 361.4 gm/mol
d) 134.2 gm/mol
Q.4 Along with antihistaminic property, oxatomide also has?
a) Anti-diabetic activity
b) Anti-neoplastic actvity
c) Anti-serotonergic activity
d) Anti-hyperlipidemic activity
Q.5 Which amongst the following is not a therapeutic use of drug oxatomide?
a) Chronic urticaria
b) Allergic rhinitis
c) Hay fever
d) Anticoagulant
Q.6 Which of the following drug and their classification are correct?
I. Oxatomide: H1 receptor antihistamine drug
II. Famotdine: H2 receptor antihistamine drug
III. Pantoprazole: Proton pump inhibitor
IV. Tolbutamide: Antineoplastic drug
a) I, III
b) I, II, III, IV
c) III, IV
d) I, II, III
Q.7 1-(3-chloropropyl)-2-benzimidazolone reacts with benzhydrylpiperizine in presence of sodium carbonate to give which drug?
a) Oxatomide
b) Lasoprazole
c) Amphetamine
d) Busulfan
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
ANSWERS
1-b
2-b
3-a
4-c
5-d
6-d
7-a
REFERENCES
[1] BE 852 405 (Janssen; appl. 14.3.1977; USA-prior. 21.12.1976, 2.4.1976).