SULINDAC Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
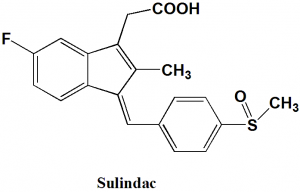
Sulindac
IUPAC nomenclature
{(1Z)-5-fluoro-2-methyl-1-[4-(methylsulfinyl)benzylidene]-1H-indene-3-yl}acetic acid
Classification
- Nonsteroidal anti-inflammatory drug
- Arylalkanoic class member
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 356.4 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | 183°C |
| 4 | Solubility | 3000 mg/L |
| 5 | Octanol/water partition coefficient | 3.42 |
| 6 | Presence of ring | Indene, phenyl |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
- Sulindac inhibits both COX-1 and COX-2 enzymes which further leads to the inhibition of prostaglandin synthesis. This is responsible for the anti-inflammatory action of the drug.
- The antipyretic action of the drug is due to action of drug on hypothalamus which results in increased blood flow, vasodilation and subsequent heat dissipation.
Structure Activity Relationship
SAR of Sulindac can be summarized as follows:
- Replacement of the indole ring with indene ring results in decrease in the CNS and GI side effects, but also the solubility in water decreases.
- On replacing the N-p-chlorobenzoyl substituent with a benzylidine function gives an active derivative.
- Analgesic effects of drug are increased on replacing the 5-methoxy group of indene with fluorine.
- Water solubility can be increased by replacing the chlorine of the phenyl substituent with sulfinyl group.
- Z-isomer is more potent than E-isomer.
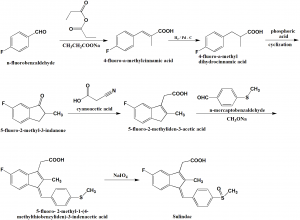
Method of synthesis
i. Condensatio of n-fluorobenzaldehyde with propionic acid in the presence of sodium acetate give 4-fluoro-α-methylcinnamic acid.
ii. On reducing the double bond of the above formed compound by palladium on carbon catalyst, 4-fluoro-α-methyldihydrocinnamic acid is produces.
iii. Resultant undergoes cyclization in the presence of polyphosphoric acid to ive 5-fluoro-2-methyl-3-indanone.
iv. The last undergoes a knowvwnagel reaction with cyanoactic aid followed by decarboxylation to produce 5-fluoro-2-methyliden-3-acetic acid.
v. On condensation of the above formed compound with n-mercaptobenzaldehyde in the presence of sodium methoxide produces 5-fluoro-2-ethyl-1-(4-methylthiobenzyliden)-3-indeaetic acid.
vi. In the final step, the sulfur atom is oxidized by sodium periodate into sulfoxide o give Sulindac. [2]
Therapeutic Uses
Sulindac is used for:
- Reducing pain, joint stiffness and swelling due to arthritis.
- Treatment of arthritis of the spine
- Treatment of gouty arthritis
- Treatment of shoulder bursitis
- Treatment of shoulder tendonitis
Side Effects
Side effects of sulindac are:
- Nausea
- Vomiting
- Gas
- Diarrhea
- Upset stomach
- Dizziness
- Headache
- Hypertension
- Ringing in ears
- Mood changes
- Painful swallowing
- Symptoms of heart failures
- Kidney problems
- Stiff neck
- Liver disease
- Allergic reactions
MCQs
Q.1 Match the following with correct SAR of the drug Sulindac.
| i. Replacement of indole ring with indene ring results in | A. Increase in CNS and GI side effects |
| ii. Z-isomer is | B. Decrease in CNS and GI side effects |
| C. More potent than E-isomer | |
| D. Less potent than E-isomer |
a) i-A, ii-C
b) i-A, ii-D
c) i-B, ii-C
d) i-B, ii-D
Q.2 Correct sequence for the True/False for correct IUPAC names of the drug can be?
- Sulindac: (N-Methyl-8-azabicyclo[3.2.1]oct-3-yl) 2-hydroxy-2-phenylacetate
- Morphine: (2-chlorophenyl)-3-hydroxy-1,3-dihydro-1,4-benzodiazepin-2-one.
- Benztropin: {(1Z)-5-fluoro-2-methyl-1-[4-(methylsulfinyl)benzylidene]-1H-indene-3-yl}acetic acid
- Phenytoin: ethyl 1-(3-cyano-3,3-diphenylpropyl)-4-phenylpiperidine-4-carboxylate
a) FFFF
b) FTFT
c) TFFT
d) FTTF
Q.3 Octanol/water partition coefficient of Sulindac is?
a) -2
b) -3.42
c) 2
d) 3.42
Q.4 Correct statement/s related with the mechanism of action of drug sulindac is/are?
a) It inhibits COX-1 enzymes
b) It inhibits COX-2 enzymes
c) It produces action on hypothalamus to produce antipyretic action
d) All of the above
Q.5 Which amongst the following is NOT a Medicinal use of drug sulindac?
a) As a general anesthesia
b) Reducing pain due to arthritis
c) Treatment of shoulder Bursitis
d) Treatment of shoulder tendonitis
Q.6 Which of the following drug and their classification are correct?
I. Sulindac: Nonsteroidal anti-inflammatory drug
II. Risperidone: Nitrogen mustard
III. Hyoscyamine: Cytotoxic agent
IV. Glutemide: epipodophyllotoxin drug
a) I, III, IV
b) I
c) II, III
d) I, II, III, IV
Q.7 Number of chitral carbons present in the structure of Sulindac?
a) 0
b)1
c) 2
d) 3
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-c
2-a
3-d
4-d
5-a
6-b
7-a