Thin layer Chromatography: Mobile Phase, Activation of TLC Plates, Development, Detecting agent and MCQ
Practical Requirements :-
- Stationary phase
- Glass plate
- Preparation and activation of TLC plates
- Application of sample
- Development of tank
- Mobile phase
- Development of technique
- Detecting agent or visualizing agent
Among them Stationary phase already discussed in previous article
Now discuss further
2. Glass plates :-
Glass plate which are specific dimensional like 20 × 20 cm ( full plate), 20×10 cm ( half plate), 20 × 5 cm (Quarter plate) can be used.
3. Preparation and activation of TLC plates:-
Preparation of the TLC plate :- there are mainly four method
1. Pouring :- The adsorbent is made into an slurry and is poured into a glass plate which is kept on a level surface.
The slurry is spread uniformly over the surface and is dried in on oven.
2. Dipping :- whole plate is dipped in slurry.
3. Spraying :- The adsorbent is made into a suspension and is sprayed on a glass plate using a sprayer.
4. Spreading :- The adsorbent is made into a slurry and is placed in an applicator and is spreaded on a plate by means of moving the applicator or plate.
4. Activation of adsorbent layer :-
Since water or other polar solvent greatly affect the development by adsorption chromatography they should be removed from the adsorbent layer. plate can be activated by placing in an oven at 120°-130° C° for about 30 minutes.
5. Mobile phase :-
Ideal requirements of mobile phase:-
- It should be safe.
- It should be non toxic.
- It should be easily available.
- It is not destory sample.
- Chemically inert.
As per ICH guide lines mainly three types of the solvent
Class-I
Class -II
Class – III
In normal phase separation non polar solvent like hexane, toluene, cyclohexane are useful and for reverse phase, polar solvent give effective separation.
6. Sample Application:-
Sample solution containing analyte mixture is dissolved in suitable solvent and applied 1-2 mm from the edge of the plate.
Sample is applied with the help of fixed volume disposable capillaries or micropipette. For best separation efficiency the spot diameter should be not more than 5 mm.
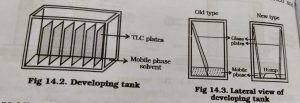
7.Development of tank :-
By development of the tank we are using less amount of mobile phase and use more number of TLC plates.
When anew Method is developed,it is better to develop in glass beakers, specimen jar, etc to avoid more wastage of solvent.
New type of development of the chamber in one chamber we have put two TLC plates.
Other types of new development we have more than two plates are placed in chamber.
Development of technique :-
Ascending method : Development of chromatogram is usually carried out by ascending method,by using special TLC chamber.
Chamber is allowed to saturate with solvent vapour in the atmosphere. Plate is also allowed to saturate with these solvent vapour if required by exposing the plate to solvent vapour is closed chamber. Then the plate is dipped in developing to effect separation of analyte mixture.
Horizontal, sandwich,two dimensional technique are also use in TLC.
Visualisation :-
After the development of TLC plates, the spot should be visualized.
Detection of spot can be done by two Method
A. Non specific method :- It is used when the number of spot can be detected but not the exact nature or type of compound.
Example 1. Iodine chamber :– where brown or Amber spots are observed when the TLC plates are kept in a tank with few iodine crystal at the bottom.
2. UV chamber for flourescent compound :- when compound are view under uv chamber at 254nm or at 365 nm fluroscence can be detected.
3. Using fluroscence stationary phase
4. Sulphuric acid spray reagents.
B. Specific method :- specific spray reagents or detecting agent or visualising agent are used to find out the nature of compound or for identification purpose.
Example are
- Ferric chloride – for phenolic compound
- Ninhydrin in Acetone – Amino acid
- 3,5 – Dinitro benzoic acid – for cardiac glycoside.
Identification of solute :-
Similar to paper Chromatography Rf value can be Calculated. Sometime Rf value are preferred to be express as hrf. It is obtained by multiplying RF value by 100 e.g.0.15 RF multiple by 100 give hrf value 15 which is convenient to report.
These observation can be useful for qualitative or quantitative.
Application of TLC :-
- TLC can be successful used for isolation of vitamin like A,D,andE using silica gel.
- Tetracycline has been separated in silica gel.
- Neomycin sulphate can be isolating on activated compound.
- Amino acid protein and peptide can be isolated and recovery by using silica gel as an adsorbent.
- Identification of drug.
- To detect decomposition product in drug.
MCQ.
1. Which is not TLC development ?
A. Sandwich method
B. Horizontal
C. Teo dimensional
D. Column
2. Which is not an application of TLC ?
A.TLC can be successful used for isolation of vitamin like A,D,andE using silica gel.
B. Identification of drug.
C.Neomycin sulphate can be isolating on activated compound.
D. To determination of moisture.
3. In Iodine chamber, which colour spot are seen ?
A. Red colour.
B. Brown colour.
C. Yellow colour
D. Green colour
Answer key
1. D
2. D
3. B
MCQ Part 2
1. Half plate size is
A. 20×10 cm
B. 10× 10 cm
C. 20× 20 cm
D. 5×5 cm
2. Which method are used for suspension spraying ?
A. Pouring
B. Dipping
C. Spraying
D. Spreading
3. Capillary diameter is
A. 5 mm
B. 6 mm
C. 7 mm
D. 10 mm
4. Which temperature is required for activation of plate ?
A. 110-120 C°
B. 120-130 C°
C. 130-149 C°
D. 150-160 C°
5. Which is not Requirement of mobile phase?
A. It should be not toxic.
B. It should be easily available.
C. It should be chemically inert.
D. It should be chemically active.
6. How many time is required to dry TLC plate?
A. 15 minutes
B. 30 minutes
C. 10 minutes
D. 7 minutes
Answer key MCQ part 2
1. A
2. C
3. A
4. B
5. D
6. B
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Reference:-
TEXT BOOK OF PHARMACEUTICAL ANALYSIS THIRD EDITION OF DR. RAVI SANKAR (14.1 TO 14.2)
TEXT BOOK OF PHARMACEUTCAL ANALYSIS FOUR EDITION OF DAVID.G.WATSON (351 TO 365)