NIFEDIPINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
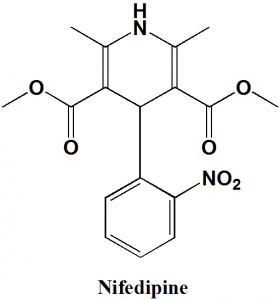
Nifedipine
IUPAC nomenclature
3,5-dimethyl 2,6-dimethyl-4-(2-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
Classification
- Dihydropyridines
- Calcium channel blockers
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 346.3 g/mol |
| 2 | Physical appearance | Yellow crystals or powder |
| 3 | Melting point | 173oC |
| 4 | Solubility | 250 gm/L in acetone |
| 5 | Octanol/water partition coefficient | 2.2 |
| 5 | Presence of ring | Dihydropyridine, phenyl |
| 6 | Number of chiral centers | Not present |
Mechanism of Action
i. Nifedipine block the voltage gated L-type Ca+2 channels in the myocardial cells and vascular smooth muscles.
ii. Due to this, the entry of calcium ions during depolarization is prevented.
iii. Resulting reduced peripheral arterial vascular resistance and dilating the coronary arteries.
iv. This helps in reducing the blood pressure and increasing the oxygen supply to the heart and helps to lessen the angina.
Structure Activity Relationship
General structure activity of dihydropyridines calcium channel blockers can be summarized as:
- R1 should be unsubstituted.
- R1 should be easily detachable group.
- Basic amino ethyl ether chain at R2 increases the potency of drug, while H/aryl group results in loss of activity of drug.
- Substitution of R3 and R5 with alkoxy carbonyl group gives optimum activity.
- Branching of alkyl chain of ester group decreases the activity.
- R4 must be phenyl ring.
- S-enantiomers are more active than R-enantiomers.
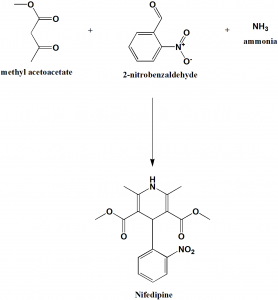
Method of synthesis
2 molecules of methylacetoacetate, 1 molecule of 2-benzaldehyde and and ammonia react together to give nifedipine drug. [1]
Medicinal Uses
Nifedipine is used for the treatment of:
- Vasopastic angina
- Chronic stable angina
- Hypertension
Side Effects
Side effects of Nifedipine are:
- Dizziness
- Weakness
- Flushing
- Fainting
- Lightheadedness
- Swelling of ankles
- Constipation
- Headache
- Allergic reactions
MCQs
Q.1 Mechanism of action of nifedipine includes?
a) Blockking of L-type calcium channels
b) Increasing the active reabsorption of calcium and chloride in PCT
c) Alkylation of genetic material in cell
d) Preventing of the active reabsorption of calcium and chloride in PCT
Q.2 Therapeutic use of drug nifedipine is/are?
a) Treatment of vasoprastic angina
b) Treatment of Hypertension
c) Treatment of stable angina
d) All of the above
Q.3 Which of the following statement is correct related with the SAR of dihydropyridines calcium channel blockers?
a) Branching of alkyl chain of ester group increases the activity
b) Subtitution of R3 with alkoxy group decreases the activity
c) S-enantiomers are more active than R-enantiomers
d) All of the above
Q.4 Nifedipine can be synthesized by the reaction between 2-benzaldehyde, ammonia and?
a) Methylacetoacetate
b) 3,5-disulfonamido-5-trifluoromethylaniline
c) Amyl alcohol
d) Propanone
Q.5 Correct sequence for the True/False for the physiochemical properties of the drug nifedipine can be?
I. Molecular weight = 346.3 gm/mol
II. Physical appearance = Colorless to yellowish liquid
III. Melting point = 173 oC
a) TFT
b) FFT
c) FFF
d) TTT
Q.6 Correct statements for the IUPAC nomenclatures of the drug are?
I. Nifedipine: 3,5-dimethyl 2,6-dimethyl-4-(2-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
II. Pentoprazole: (RS)-6-(Difluoromethoxy)-2-[(3,4-dimethoxypyridin-2-yl)methylsulfinyl]-1H-benzo[d]imidazole
III. Buclizine: (RS)-1-[(4-chlorophenyl)- phenyl-methyl]-4- [(4-tert-butylphenyl) methyl] piperazine
IV. Cimetidine: 1-cyano-2-methyl-3-[2-[(5-methyl-1H-imidazol-4-yl)methylsulfanyl]ethyl]guanidine
a) I, II, III, IV
b) III, IV
c) II, III, IV
d) I, II
Q.7 Match the following drugs with their correct classifications-
| i. Nifedipine | A. Calcium channel blocker |
| ii. Omeprazol | B. Antimetabolite |
| iii. Thioguanine | C. Antihyperlipidemic |
| iv. Cholestipol | D. Proton pump inhibitor |
a) i-A, ii-C, iii-D, iv-B
b) i-A, ii-D, iii-B, iv-C
c) i-D, ii-B, iii-A, iv-C
d) i-D, ii-A, iii-C, iv-B
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
ANSWERS
1-a
2-d
3-c
4-a
5-a
6-a
7-b
REFERENCES
[1] Vardanyan R, Hruby V. Synthesis of essential drugs. Elsevier; 2006 Mar 10.