Carbohydrates: Classification, Chemical nature, Biological role, digestion and absorption and MCQs
Carbohydrates are defined as aldehyde or ketone derivative of polyhydroxy ( more than one OH group)alcohols; or compounds that form these derivatives on hydrolysis. Carbohydrates are also known as saccharides. The empirical formula of many simple carbohydrates is [CH2O]n; while other carbohydrates like deoxyribose, rhamnohexos do not show this formula.
Functions of carbohydrates include :-
-
- source of energy for living beings ex- glucose
- it serves as a structural component like glycosaminoglycans in humans, cellulose in plants and chitin in insects.
- constituent of nucleotide which further forms DNA and RNA.
- plays important role in lubrication, cellular intercommunication and immunity.
- carbohydrates like glucuronic acid is involved in detoxification reaction.
Carbohydrates are classified in 3 main groups:-
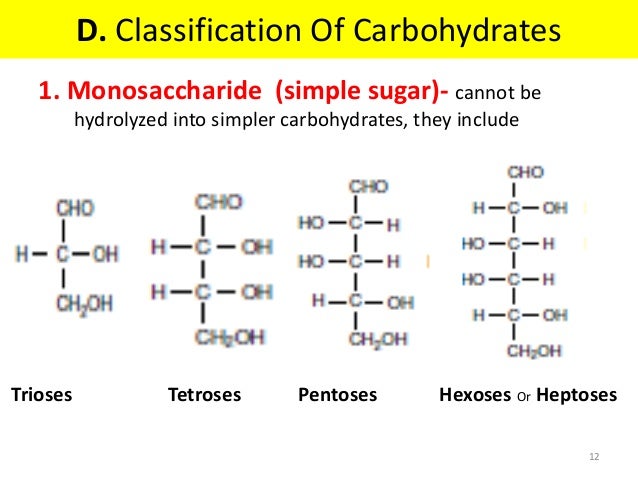
1. Monosaccharides
also known as simple sugars. Carbohydrates are termed as sugars when they are soluble in water and sweet in taste. Monosaccharides consist of a single polyhydroxy aldehyde or ketone group. These are further subdivided on two basis
- depending upon no. of carbon atoms present:- example: trioses, tetroses, pentoses, hexoses heptoses
- depending on the functional group present:- like ketoses or aldoses.
Chemical properties of monosaccharides involve:-
- action of strong acid
- action of alkalis
- oxidation: sugar acid formation
- reduction: sugar alcohol formation
- action of phenyl-hydrazine.
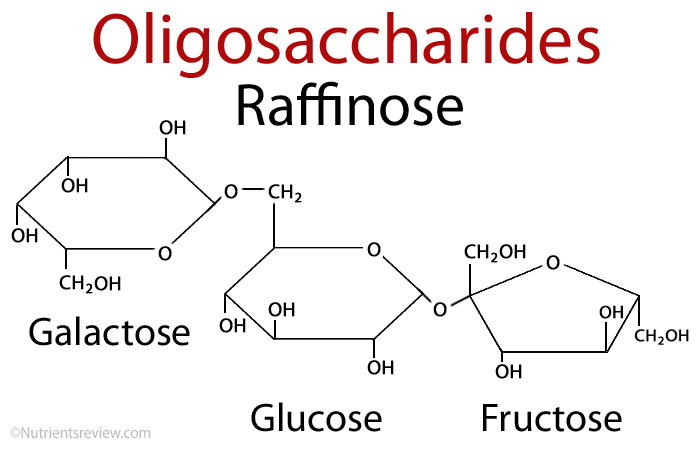
2. Oligosaccharides:
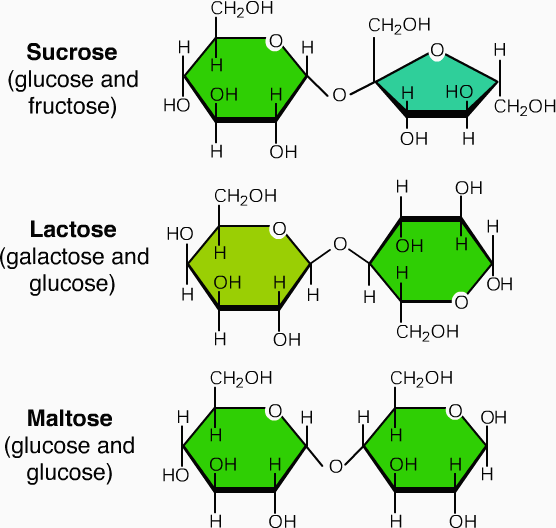
It consist of short chains of monosaccharide units, which are are joint together by bond known as glycosidic bond. On hydrolysis it gives two to ten molecules of simple sugar. Oligosaccharides are divided according to the no. of monosaccharide units present in them; these are described in below given table.
| Types of oligosaccharides | No. of monosaccharides | Example | Types of monosaccharide present |
| Disaccharides | two | Maltose
Lactose sucrose |
Glucose+ glucose
Glucose+ galactose Glucose+ fructose |
| Trisaccharide | three | raffinose | Glucose+ galactose+ fructose |
| Tetrasaccharide | four | stachyose | 2 galactose+ glucose +fructose |
oligosaccharides with more than three subunits are usually found bound as side chains in glycoproteins.
3. Polysaccharides
These are the polymers consisting of 100-1000 molecules of monosaccharides. They are also known as glycans or complex carbohydrates.
Physical properties of polysaccharides are:-
- They are either branched or linear in structure.
- high molecular weight
- sparingly soluble in cold water
- form colloidal solution when heated with water
- non sweetish in taste
- do not exhibit any properties of aldehyde or ketone group.
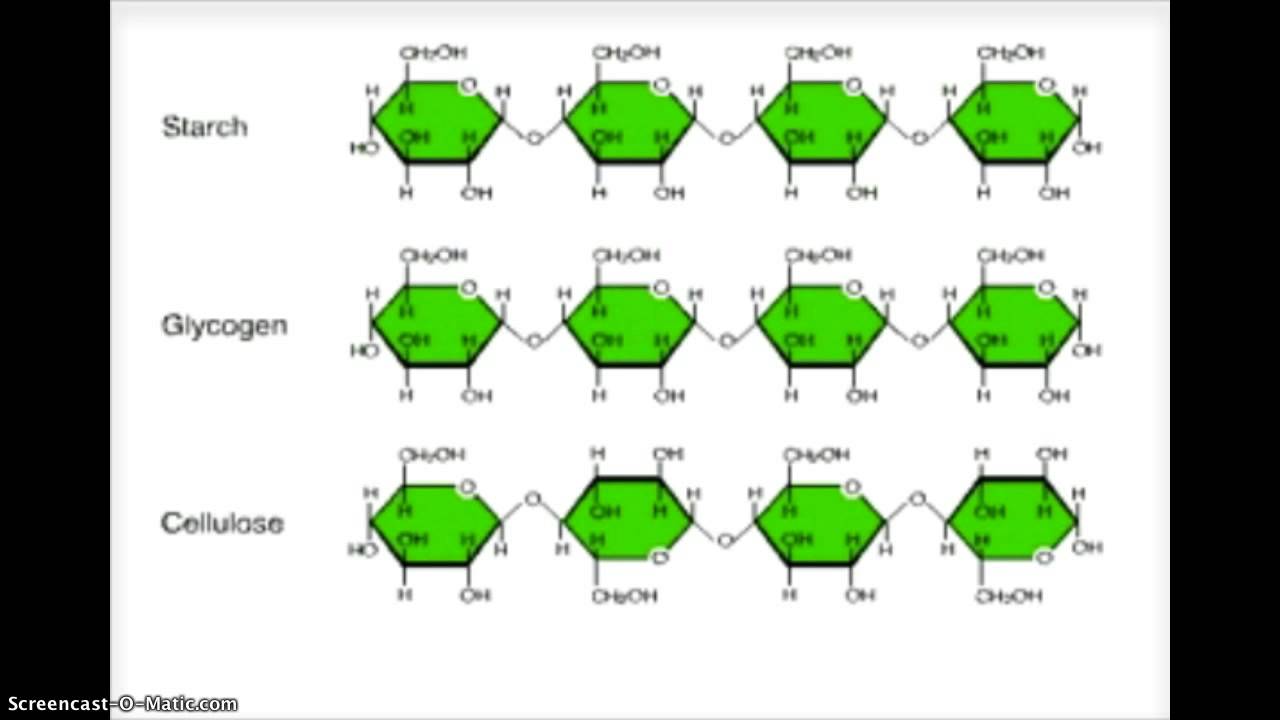
Polysaccharides are of two types:
- Homo-polysaccharides:- also known as homoglycans, these are made up of several units of the same one type of monosaccharide. Examples are- starch, dextrins, glycogen, dextrans, insulin and cellulose.
- Hetero-polysaccharides:- also known as heteroglycans; these are made up of two or more different types of monosaccharide units. Hetero-polysaccharide in plants are agar, gum, pectins etc. While these in human beings are heparin, chondroitin, hyaluronic acid etc.
DIGESTION AND ABSORPTION OF CARBOHYDRATES
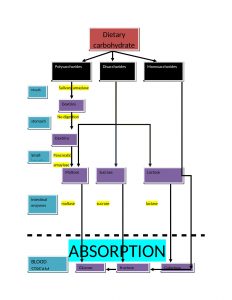
Key points for digestion and absorption are as follows:
- The main site of absorption is mouth and small intestine. Dietary carbohydrates are polysaccharides(glycogen, starch and cellulose), disaccharides(sucrose and lactose) and monosaccharide(glucose and fructose).
- monosaccharide needs no digestion before absorption but disaccharides and polysaccharides are firstly converted in simple form before their absorption takes place.
- hydrolysis of starch and cellulose is initiated by salivary amylase in mouth.
- In stomach, the digestion process is put on hold because the high acidity inactivates the salivary amylase
- there are two phases of digestion in intestine: firstly due to pancreatic alpha amylase and secondly due to enzymes of brush border
- Two mechanisms for the absorption of monosaccharides are
a. active transport: works against the concentration gradient
b. facilitative transport: with concentration gradient.
Multiple choice questions(MCQs)
1. Which of the following are also known as sugars?
A. fats B. lipids
C. vitamins D. carbohydrates
2. Which process is responsible for absorption of carbohydrates
A. active transport B. simple diffusion
C. facilitative transport D. both A and C
3. Where does salivary amylase becomes inactivated?
A. mouth B. stomach
C. small intestine D. all of the above
4. Match the following-
a. monosaccharide 1. lactose
b. heteroglycans 2. starch
c. homoglycans 3. Gum
d. disaccharide 4. pentoses
5. Which of the following is the function of carbohydrates?
A. lubrication B. constituent of nucleotide
C. source of energy D. all of the above
6. Which of the following statement is not true?
A. digestion of carbohydrates occur in mouth and small intestine mainly
B. pancreatic amylase inactivates in stomach
C. polysaccharides are polymers of 1000 monosaccharide unit
d. the empirical formula of carbohydrates is [CH2O]n
7. which enzyme is responsible for conversion of dextrins to maltose?
A. maltase B. salivary amylase
C. pancreatic amylase D. gastric enzyme
8. Which of the following is the example of oligosaccharide?
A. starch B. trioses
C. disaccharides D. gum
9. what happens when dextrins reaches stomach?
A. converts in maltose B. converts in lactose
C. no digestion D. converts in sucrose
10. Which of the following is not the physical property of polysaccharides?
A. linear or branched in structure B. non sweetish
C. low molecular weight D. sparingly soluble in H2O
11. Which functional group is present in monosaccharides?
A. ketones B. aldehyde
C. alcohols D. both A and B
12. Which of the following is the example of homoglycans?
A. starch B. agar
C. gum D. heparin
13. which is not counted under the chemical property of monosaccharides?
A. action of alkalis B. reduction: sugar alcohol formation
C. action of strong acid D. none of the above
14. Which bond lies between the monosaccharide units to form oligosaccharides?
A. hydrogen bond B. covalent bond
C. glycosidic bond D. none of the above
15. Polysaccharides are also known as?
A. homoglycans B. simple sugars
C. heteroglycans D. glycans
ANSWERS:-
- carbohydrates
- both A and C
- stomach
- a – 4 b – 3 c – 2 d – 1
- all of the above
- pancreatic amylase inactivates in stomach
- pancreatic amylase
- disaccharides
- no digestion
- low molecular weight
- both A and B
- starch
- none of the above
- glycosidic bond
- glycans
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
REFERENCE:- Pankaja Naik- Biochemistry; 4th edition; page no:- 13-21 and 157-159