CARBOPLATIN Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Carboplatin
IUPAC nomenclature
Cis diammine(cyclobutane-1,1-dicarboxylate-O,O’)platinum(II)
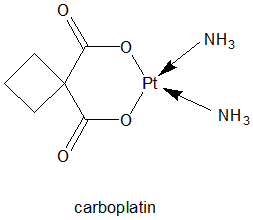
Classification
Carboplatin falls under the category of Antineoplastic cytotoxic drug. [1]
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 371.25g/mol |
| 2 | Appearance | White crystals |
| 3 | Melting point | 228-230°C |
| 4 | Solubility | Soluble in water |
| 5 | Presence of ring | Cyclobutane |
Mechanism of Action
i. It introduces the alkyl groups to the DNA. When repair enzymes tries to remove the alkylated fragments, they fragment the DNA.
iii. It binds with DNA and produces cross-linkages.
iii. It induces mispairing of the DNA causes mutations in the cells.[2]
Structural Activity Relationship
- A leaving group is necessary for the activity.
- Cytotoxicity of oxiplatin is also greater than carboplatin.
- Organic functionalities of nonleaving groups coordinated to platinum are critical for selective uptake by OTCs
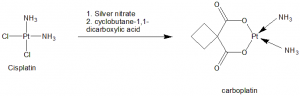
Methods of Synthesis
i. Cisplatin is reacted with silver nitrate to form cis-diamino-(1,1-cyclobutandicarboxylate)platinium(II).
ii. The above formed compound is then reacted with cyclobutan-1,1-dicarboxylic acid to form carboplatin.
Therapeutic Uses
Carboplatin is used for the treatment of:
- Ovarian cancer
- Osteogenic sarcoma
- Germ cell tumors
- CNS tumors
- Cervical cancer
- Breast cancer
- Bladder cancer
- Esophageal cancer
- Endometrial cancer
- Head and neck cancer
- Lung cancer
Carboplatin is also used for the preparation of the germ cells and during the bone marrow transplant.
Side Effects
- The common side effects of carbolatin include low blood counts, nausea, vomiting, tatste changes, loss of hair, weakness and blood test abnormalities.
- Some of the less common side effects of carboplatin are abdominal pain, diarrhea, burning sensation at injection site, constipation, mouth sores, peripheral neuropathy, central neurotoxicity, nephrotoxicity, ototoxicity, abnormal blood electrolyte levels, abnormal blood liver enzymes, some cardiovascular abnormalities and allergic reactions.
MCQs
Q.1 Which term is associated with the drug carboplatin?
a) Kemocarb
b) Oncocarbin
c) Shantinib
d) Both a) and b)
Q.2 Match the following with respect to the physical forms of drugs
| i. Carboplatin | A. Ivory microcrystalline solid |
| ii. Dacarbazine | B. Orange yellow solid |
| iii. Methotrexate | C. White crystalline form |
| iv. Carmustine | D. Yellow to brown crystalline powder |
a) i-C, ii-A, iii-D, iv-B
b) i-A, ii-D, iii-B, iv-C
c) i-C, ii-B, iii-A, iv-D
d) i-B, ii-A, iii-C, iv-D
Q.3 The drug Carboplatin shows its action through?
a) Introduction of tyrosyl group
b) Alkylation of DNA
c) Interacting with cellular enzymes
d) None of the above
Q.4 The correct order for the synthesis of the drug carboplatin is?
I. Reaction of cisplatin with silver nitrate
II. Reaction of cisplatin with Potassium dichromate.
III. Reaction with cyclobutan-1,1-dicarboxylic acid.
IV. Reaction with cyclopropane-1,1-dicarboxylic acid.
a) I – III
b) I – IV
c) II – III
d) II – IV
Q.5 Predict the incorrect statements from the following with respect to the classification of the drug.
I. Vincristine is an epipodophyllotoxin
II. Cytarabine is a pyrimidine antagonist.
III. Carboplatin is an antineoplastic drug.
IV. Flutamide is an antiandrogen.
a) I, II & III
b) I only
c) III and IV
d) None
Q.6 The correct sequence of True and False for the given statements with repects to the side effects of drug Carboplatin is?
I. Low blood count is a common side effect.
II. Blood tests abnormalities may occur.
III. Central neurotoxicity is a less common side effect.
IV. Hearing loss may occur
a) TTTT
b) FFFF
c) TFTF
d) TTFT
Q.7 Which amongst the following drugs is having highest number of ring system in its structure-
a) Cisplatin
b) Dacarbazine
c) Methotrexate
d) Carboplatin
ANSWERS
1-d
2-a
3-b
4-a
5-b
6-a
7-c